Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for a Cost-Effective and Dynamic Biomarker
Abstract
1. Introduction
2. Results
2.1. Patient’s Characteristics
2.2. Prognostic Role of Pre-Treatment Plasmatic LDH
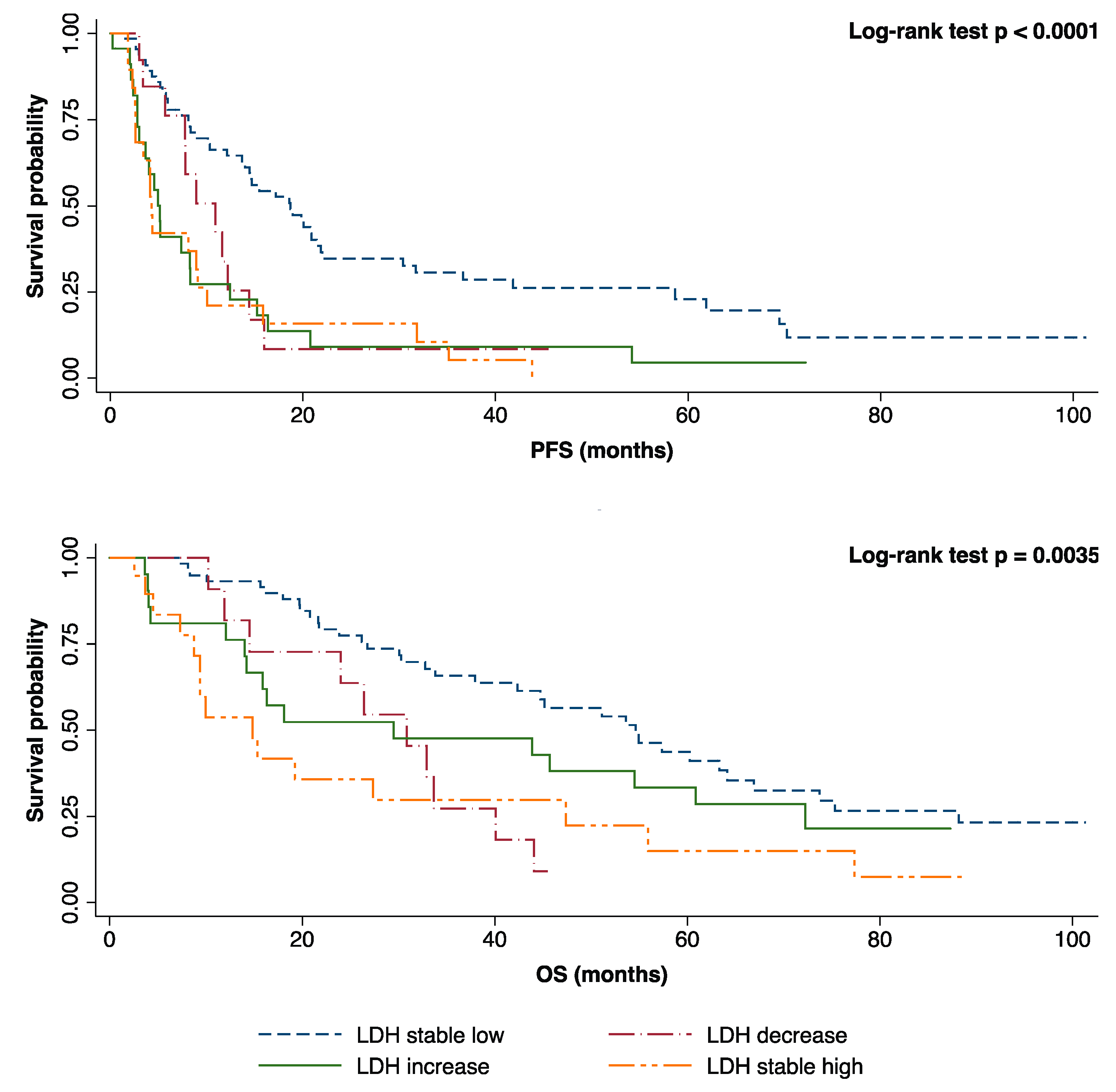
2.3. Prognostic Role of Plasmatic LDH Response during First-Line Treatment.
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Source
4.3. Blood Sample Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures 2018; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of Outcome in Metastatic Breast Cancer: Insights From a Real-World Scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Petekkaya, I.; Unlu, O.; Roach, E.C.; Gecmez, G.; Okoh, A.K.; Babacan, T.; Sarici, F.; Keskin, O.; Arslan, C.; Petekkaya, E.; et al. Prognostic role of inflammatory biomarkers in metastatic breast cancer. J. B.U.ON. 2017, 22, 614–622. [Google Scholar]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat. Rev. 2019. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Hayes, D.F.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Reuben, J.M.; Doyle, G.V.; Matera, J.; Allard, W.J.; Miller, M.C.; et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 2005, 23, 1420–1430. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Q.; Pan, Y.; He, B.; Ying, H.; Sun, H.; Liu, X.; Wang, S. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer. FEBS Open Bio. 2015, 5, 502–507. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Wu, C.; Zhang, L.; Mei, Q.; Hu, G.; Long, G.; Sun, W. Prognostic significance of serum lactate dehydrogenase in patients with breast cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 3611. [Google Scholar] [CrossRef]
- Chen, B.; Dai, D.; Tang, H.; Chen, X.; Ai, X.; Huang, X.; Wei, W.; Xie, X. Pre-treatment serum alkaline phosphatase and lactate dehydrogenase as prognostic factors in triple negative breast cancer. J. Cancer 2016, 7, 2309. [Google Scholar] [CrossRef]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase a in cancer: A promising target for diagnosis and therapy. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef]
- Gallo, M.; Sapio, L.; Spina, A.; Naviglio, D.; Calogero, A.; Naviglio, S. Lactic dehydrogenase and cancer: An overview. Front. Biosci. (Landmark Ed.) 2015, 20, 1234–1249. [Google Scholar]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Lactate dehydrogenase 5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015, 358, 1–7. [Google Scholar] [CrossRef]
- Garcia, R.; Hernandez, J.M.; Caballero, M.D.; Gonzalez, M.; Galende, J.; Del Cainizo, M.C.; Vazquez, L.; San Miguel, J.F. Serum lactate dehydrogenase level as a prognostic factor in hodgkin’s. Br. J. Cancer 1993, 68, 1227. [Google Scholar] [CrossRef][Green Version]
- Ferraris, M.; Giuntini, P.; Gaetani, G.F. Serum Lactic Ddehydrogenase as a prognostic tool for Non-Hodgkin Lymphomas. Blood 1979, 54, 928–932. [Google Scholar]
- Gkotzamanidou, M.; Kastritis, E.; Roussou, M.; Migkou, M.; Gavriatopoulou, M.; Nikitas, N.; Gika, D.; Mparmparousi, D.; Matsouka, C.; Terpos, E.; et al. Increased serum lactate dehydrongenase should be included among the variables that define very-high-risk multiple myeloma. Clin. Lymphoma, Myeloma Leuk. 2011, 11, 409–413. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, F.; Yang, S.; Hua, L.; Wu, J.; Zhan, W. The Clinicopathologic Importance of Serum Lactic Dehydrogenase in Patients with Gastric Cancer. Dis. Markers 2014. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199. [Google Scholar] [CrossRef]
- Hermes, A.; Gatzemeier, U.; Waschki, B.; Reck, M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer—A retrospective single institution analysis. Respir. Med. 2010, 104, 1937–1942. [Google Scholar] [CrossRef]
- Lee, D.S.; Park, K.R.; Kim, S.J.; Chung, M.J.; Lee, Y.H.; Chang, J.H.; Kang, J.H.; Hong, S.H.; Kim, M.S.; Kim, Y.S. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: Predictive value of metastases and relation to survival outcomes. Tumor Biol. 2016, 37, 619–625. [Google Scholar] [CrossRef]
- Naruse, K.; Yamada, Y.; Aoki, S.; Taki, T.; Nakamura, K.; Tobiume, M.; Zennami, K.; Katsuda, R.; Sai, S.; Nishmo, Y.; et al. Lactate dehydrogenase is a prognostic indicator for prostate cancer patients with bone metastasis. Acta Urol. Jpn. 2007. [Google Scholar]
- Scartozzi, M.; Giampieri, R.; MacCaroni, E.; Del Prete, M.; Faloppi, L.; Bianconi, M.; Galizia, E.; Loretelli, C.; Belvederesi, L.; Bittoni, A.; et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br. J. Cancer 2012, 106, 799. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Z.; Zhuang, Q.; Fan, M.; Ding, T.; Lu, H.; He, X. Prognostic value of serum lactate dehydrogenase in renal cell carcinoma: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166482. [Google Scholar] [CrossRef]
- Ryberg, M.; Nielsen, D.; Øterlind, K.; Skovsgaard, T.; Dombernowsky, P. Prognostic factors and long-term survival in 585 patients with metastatic breast cancer treated with epirubicin-based chemotherapy. Ann. Oncol. 2001, 12, 81–87. [Google Scholar] [CrossRef]
- Pierga, J.Y.; Asselain, B.; Jouve, M.; Diéras, V.; Carton, M.; Laurence, V.; Girre, V.; Beuzeboc, P.; Palangié, T.; Dorval, T.; et al. Effect of adjuvant chemotherapy on outcome in patients with metastatic breast carcinoma treated with first-line doxorubicin-containing chemotherapy. Cancer 2001, 91, 1079–1089. [Google Scholar] [CrossRef]
- Yamamoto, N.; Watanabe, T.; Katsumata, N.; Omuro, Y.; Ando, M.; Fukuda, H.; Tokue, Y.; Narabayashi, M.; Adachi, I.; Takashima, S. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J. Clin. Oncol. 1998, 16, 2401–2408. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Q.H.; Ye, Y.; Hildebrandt, M.A.T.; Gu, J.; Wu, X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with nonmetastatic breast cancer. Carcinogenesis 2014, 36, 243–248. [Google Scholar] [CrossRef]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 115–124. [Google Scholar]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomarkers 2017, 19, 353–363. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018, 28, 848–865. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, J.; Wang, Z.; Wang, B.; Wang, L.; Cao, J.; Tao, Z.; Hu, X. An explorative analysis of the prognostic value of lactate dehydrogenase for survival and the chemotherapeutic response in patients with advanced triple-negative breast cancer. Oncotarget 2018, 9, 10714. [Google Scholar] [CrossRef]
- Dong, T.; Liu, Z.; Xuan, Q.; Wang, Z.; Ma, W.; Zhang, Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci. Rep. 2017, 7, 6069. [Google Scholar] [CrossRef]


| Characteristics | Number of Patients (Total = 392) | % | |
|---|---|---|---|
| Age | <45 years | 42 | 10.71 |
| 45–65 years | 182 | 46.43 | |
| >65 years | 168 | 42.86 | |
| Menopausal state | Pre-menopausal | 114 | 29.08 |
| Post-menopausal | 233 | 59.44 | |
| Unknown | 45 | 11.48 | |
| Histotype | Ductal | 315 | 80.36 |
| Lobular | 59 | 15.05 | |
| Other | 12 | 3.06 | |
| Unknown | 6 | 1.53 | |
| Profile | Luminal A | 44 | 11.22 |
| Luminal B | 150 | 38.27 | |
| Luminal HER2 | 43 | 10.97 | |
| HER2-positive | 34 | 8.67 | |
| Triple negative | 37 | 9.44 | |
| Unknown | 84 | 21.43 | |
| ECOG PS | 0 | 201 | 51.28 |
| 1 | 150 | 38.26 | |
| ≥2 | 34 | 8.67 | |
| Unknown | 7 | 1.79 | |
| Number of metastatic sites | 1 | 212 | 54.08 |
| 2 | 104 | 26.53 | |
| ≥3 | 76 | 19.39 | |
| Site of metastases * | Bone | 199 | 50.77 |
| Bone only | 79 | 20.15 | |
| Liver | 99 | 25.26 | |
| CNS | 25 | 6.38 | |
| Lung | 110 | 28.06 | |
| Lymph nodes | 133 | 33.93 | |
| Firs-line treatment | Chemotherapy | 231 | 58.93 |
| Hormonal therapy | 161 | 41.07 | |
| Baseline LDH level | High 1 | 69 | 17.60 |
| Normal | 150 | 38.27 | |
| Unknown | 173 | 44.13 | |
| Baseline ALP level | High 2 | 124 | 31.63 |
| Normal | 245 | 62.50 | |
| Unknown | 23 | 5.87 | |
| Covariates | Number of Patients | Univariate Analysis(HR, 95% CI) | p | Multivariate Analysis(HR, 95% CI) | p | |
|---|---|---|---|---|---|---|
| Age | <45 years | 42 | 0.80 (0.54–1.17) | 0.25 | ||
| 45–65 years | 182 | Ref. | - | |||
| >65 years | 168 | 0.95 (0.75–1.20) | 0.68 | |||
| Profile | Luminal A | 44 | Ref. | - | Ref. | - |
| Luminal B | 150 | 1.27 (0.87–1.87) | 0.20 | 1.10 (0.66–1.84) | 0.68 | |
| Luminal HER2 | 43 | 0.73 (0.44–1.19) | 0.21 | 0.58 (0.31–1.10) | 0.09 | |
| HER2-positive | 34 | 1.17 (0.71–1.92) | 0.52 | 0.92 (0.44–1.92) | 0.84 | |
| Triple negative | 37 | 3.19 (1.96–5.17) | <0.0001 | 2.81 (1.44–5.48) | 0.002 | |
| ECOG PS | 0 | 201 | Ref. | - | Ref. | - |
| 1 | 150 | 1.25 (0.98–1.58) | 0.06 | 1.35 (0.90–2.02) | 0.13 | |
| ≥2 | 34 | 1.70 (1.12–2.59) | 0.01 | 2.45 (1.18–5.07) | 0.01 | |
| Number of metastatic sites | 1 | 212 | Ref. | - | Ref. | - |
| 2 | 104 | 1.35 (1.04–1.75) | 0.02 | 1.51 (0.97–2.35) | 0.06 | |
| ≥3 | 76 | 0.97 (0.71–1.34) | 0.89 | 0.61 (0.35–1.04) | 0.07 | |
| Site of metastases * | Bone | 199 | 0.93 (0.74–1.16) | 0.54 | 1.07 (0.71–1.61) | 0.73 |
| Liver | 99 | 1.14 (0.88–1.47) | 0.29 | 0.92 (0.58–1.47) | 0.75 | |
| CNS | 25 | 1.38 (0.86–2.20) | 0.17 | |||
| Lung | 110 | 0.90 (0.70–1.16) | 0.44 | |||
| Baseline LDH level | High 1 | 69 | 1.81 (1.31–2.51) | 0.0003 | 1.51 (1.02–2.26) | 0.039 |
| Normal | 150 | Ref. | - | Ref. | - | |
| Baseline ALP level | High 2 | 124 | 1.45 (1.14–1.85) | 0.002 | 1.11 (0.73–1.66) | 0.61 |
| Normal | 245 | Ref. | - | Ref. | - | |
| Covariates | Number of Patients | Univariate Analysis(HR, 95% CI) | p | Multivariate Analysis(HR, 95% CI) | p | |
|---|---|---|---|---|---|---|
| Age | <45 years | 42 | 0.82 (0.52–1.29) | 0.41 | ||
| 45–65 years | 182 | Ref. | - | |||
| >65 years | 168 | 1.16 (0.89–1.53) | 0.25 | |||
| Profile | Luminal A | 44 | Ref. | - | Ref. | - |
| Luminal B | 150 | 1.63 (1.01–2.62) | 0.04 | 2.26 (1.16–4.38) | 0.01 | |
| Luminal HER2 | 43 | 1.14 (0.63–2.03) | 0.65 | 1.73 (0.81–3.70) | 0.15 | |
| HER2-positive | 34 | 1.61 (0.88–2.96) | 0.12 | 1.24 (0.49–3.15) | 0.64 | |
| Triple negative | 37 | 4.31 (2.45–7.59) | <0.0001 | 7.19 (3.11–16.5) | <0.0001 | |
| ECOG PS | 0 | 201 | Ref. | - | Ref. | - |
| 1 | 150 | 1.92 (1.45–2.55) | <0.0001 | 1.88 (1.18–2.99) | 0.007 | |
| ≥2 | 34 | 2.61 (1.72–3.97) | <0.0001 | 1.76 (0.84–3.70) | 0.13 | |
| Number of metastatic sites | 1 | 212 | Ref. | - | Ref. | - |
| 2 | 104 | 1.40 (1.03–1.89) | 0.02 | 2.04 (1.20–3.46) | 0.008 | |
| ≥3 | 76 | 1.36 (0.95–1.94) | 0.08 | 0.70 (0.35–1.41) | 0.32 | |
| Site of metastases * | Bone | 199 | 0.95 (0.73–1.23) | 0.74 | 1.19 (0.73–1.96) | 0.47 |
| Liver | 99 | 1.33 (1.00–1.78) | 0.046 | 0.58 (0.68–1.93) | 0.58 | |
| CNS | 25 | 2.72 (1.68–4.42) | <0.0001 | 22.05 (4.38–110.94) | 0.0002 | |
| Lung | 110 | 1.10 (0.82–1.47) | 0.51 | |||
| Baseline LDH level | High 1 | 69 | 2.22 (1.55–3.19) | <0.0001 | 1.64 (1.05–2.54) | 0.027 |
| Normal | 150 | Ref. | - | Ref. | - | |
| Baseline ALP level | High 2 | 124 | 1.84 (1.40–2.41) | <0.0001 | 1.48 (0.94–2.31) | 0.08 |
| Normal | 245 | Ref. | - | Ref. | - | |
| LDH Variation 1 | Number of Patients(Total = 126) | % | Median PFS(25–75th Percentile) | Median OS(25–75th Percentile) |
|---|---|---|---|---|
| Stable low | 69 | 54.76 | 18.71 (8.09–58.65) | 54.64 (26.76–88.18) |
| High-to-low | 15 | 11.91 | 10.92 (7.76–14.43) | 30.87 (14.53–40.08) |
| Low-to-high | 23 | 18.25 | 5.13 (2.79–12.43) | 29.49 (14.01–72.26) |
| Stable high | 19 | 15.08 | 4.27 (2.60–10.06) | 14.83 (8.78–47.38) |
| Covariates | Number of Patients | Multivariate Analysis (HR, 95% CI) PFS | p | Multivariate Analysis (HR, 95% CI) OS | p | |
|---|---|---|---|---|---|---|
| Profile | Luminal A | 44 | Ref. | - | Ref. | - |
| Luminal B | 150 | 0.88 (0.43–1.80) | 0.73 | 1.39 (0.57–3.36) | 0.46 | |
| Luminal HER2 | 43 | 0.37 (0.17–0.81) | 0.01 | 0.75 (0.30–1.86) | 0.54 | |
| HER2-positive | 34 | 1.12 (0.41–3.03) | 0.12 | 0.66 (0.18–2.40) | 0.53 | |
| Triple negative | 37 | 2.90 (1.16–7.22) | 0.02 | 7.81 (2.66–22.9) | 0.0002 | |
| ECOG PS | 0 | 201 | Ref. | - | Ref. | - |
| 1 | 150 | 1.68 (0.90–3.14) | 0.10 | 1.78 (0.84–3.79) | 0.13 | |
| ≥2 | 34 | 4.19 (1.48–11.85) | 0.006 | 2.29 (0.81–6.47) | 0.11 | |
| Number of metastatic sites | 1 | 212 | Ref. | - | Ref. | - |
| 2 | 104 | 1.86 (0.92–3.73) | 0.08 | 1.75 (0.79–3.88) | 0.16 | |
| ≥3 | 76 | 0.71 (0.32–1.56) | 0.40 | 0.67 (0.25–1.79) | 0.43 | |
| Site of metastases * | Bone | 199 | 1.46 (0.76–2.81) | 0.25 | 2.35 (1.05–5.23) | 0.036 |
| Liver | 99 | 0.88 (0.44–1.76) | 0.72 | 1.53 (0.71–3.31) | 0.26 | |
| CNS | 25 | 1223.5 (42.5–35225.6) | <0.0001 | |||
| ALP variation at 12 weeks 1 | Stable low | 20262 | Ref. | - | Ref. | - |
| High-to-low | 44 | 0.82 (0.35–1.87) | 0.63 | 0.62 (0.25–1.52) | 0.30 | |
| Low-to-high | 13 | 0.88 (0.22–3.42) | 0.86 | 2.39 (0.57–10.0) | 0.23 | |
| Stable high | 62 | 0.98 (0.45–2.12) | 0.96 | 1.24 (0.53–2.88) | 0.60 | |
| LDH variation at 12 weeks 2 | Stable low | 69 | Ref. | - | Ref. | - |
| High-to-low | 15 | 1.27 (0.50–3.23) | 0.60 | 2.35 (0.82–6.77) | 0.11 | |
| Low-to-high | 23 | 3.96 (2.00–7.82) | 0.0001 | 2.02 (0.89–4.56) | 0.08 | |
| Stable high | 19 | 2.88 (1.40–5.89) | 0.003 | 2.61 (1.16–5.86) | 0.02 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelizzari, G.; Basile, D.; Zago, S.; Lisanti, C.; Bartoletti, M.; Bortot, L.; Vitale, M.G.; Fanotto, V.; Barban, S.; Cinausero, M.; et al. Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for a Cost-Effective and Dynamic Biomarker. Cancers 2019, 11, 1243. https://doi.org/10.3390/cancers11091243
Pelizzari G, Basile D, Zago S, Lisanti C, Bartoletti M, Bortot L, Vitale MG, Fanotto V, Barban S, Cinausero M, et al. Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for a Cost-Effective and Dynamic Biomarker. Cancers. 2019; 11(9):1243. https://doi.org/10.3390/cancers11091243
Chicago/Turabian StylePelizzari, Giacomo, Debora Basile, Silvia Zago, Camilla Lisanti, Michele Bartoletti, Lucia Bortot, Maria Grazia Vitale, Valentina Fanotto, Serena Barban, Marika Cinausero, and et al. 2019. "Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for a Cost-Effective and Dynamic Biomarker" Cancers 11, no. 9: 1243. https://doi.org/10.3390/cancers11091243
APA StylePelizzari, G., Basile, D., Zago, S., Lisanti, C., Bartoletti, M., Bortot, L., Vitale, M. G., Fanotto, V., Barban, S., Cinausero, M., Bonotto, M., Gerratana, L., Mansutti, M., Curcio, F., Fasola, G., Minisini, A. M., & Puglisi, F. (2019). Lactate Dehydrogenase (LDH) Response to First-Line Treatment Predicts Survival in Metastatic Breast Cancer: First Clues for a Cost-Effective and Dynamic Biomarker. Cancers, 11(9), 1243. https://doi.org/10.3390/cancers11091243







