Meta-Enrichment Analyses to Identify Advanced Gastric Cancer Patients Who Achieve a Higher Response to S-1/Cisplatin
Abstract
1. Introduction
2. Results
2.1. Creating Individual Enrichment Scores
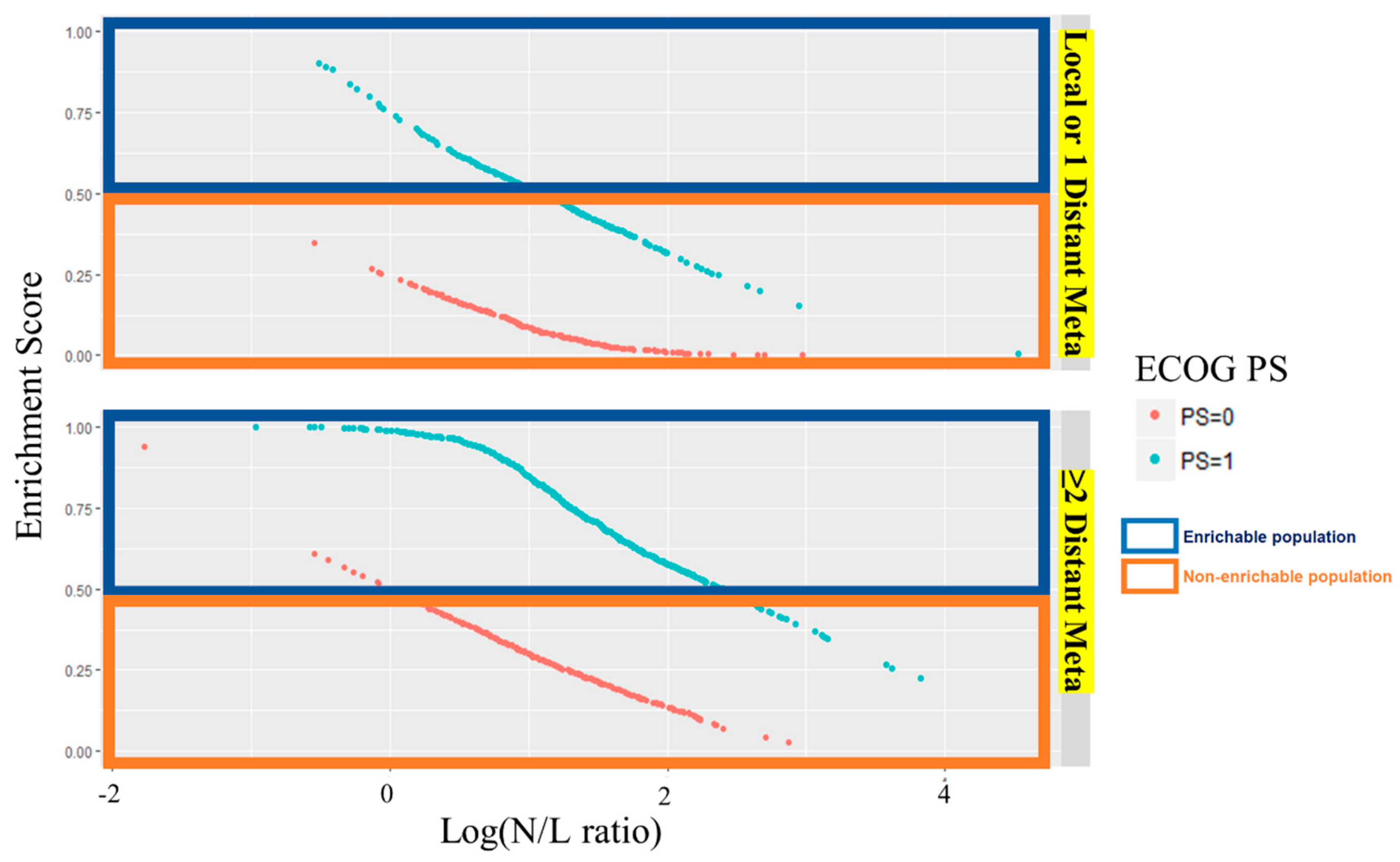
2.2. Selecting the Enrichment Subgroup
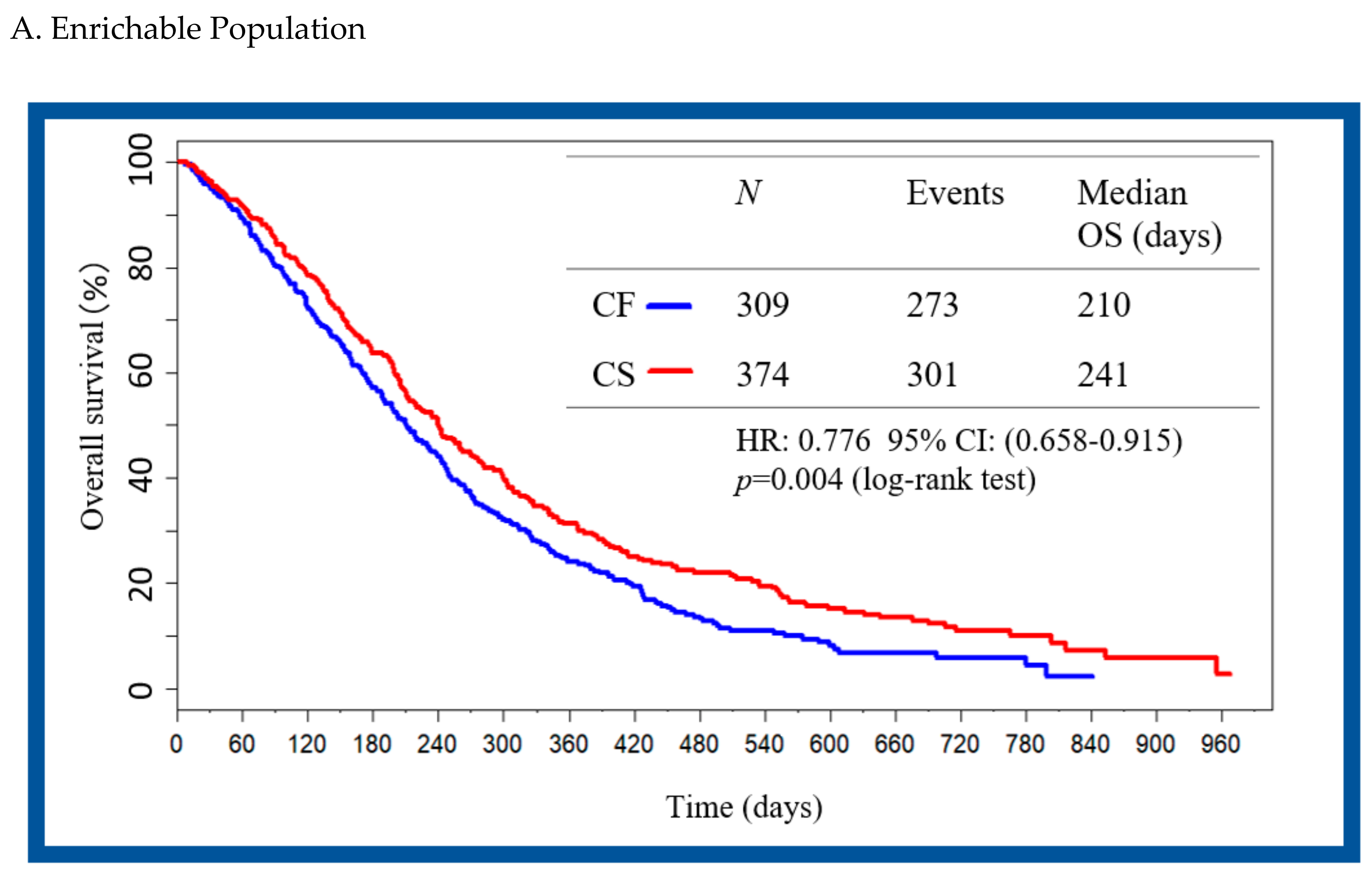
2.3. Validation Enrichment Subgroup and Patient Mapping
3. Discussion
4. Materials and Methods
4.1. Creating Individual Enrichment Scores
4.2. Selecting the Enrichment Subgroup
4.3. Validation Enrichment Subgroup and Patient Mapping
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available online: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-201 (accessed on 26 July 2018).
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011, 14, 113–123. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Gastric Cancer. Version 2. 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 26 July 2018).
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Ter Veer, E.; Haj Mohammad, N.; van Valkenhoef, G.; Ngai, L.L.; Mali, R.M.A.; Anderegg, M.C.; van Oijen, M.G.H.; van Laarhoven, H.W.M. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Rodriguez, W.; Bodoky, G.; Moiseyenko, V.; Lichinitser, M.; Gorbunova, V.; Vynnychenko, I.; Garin, A.; Lang, I.; Falcon, S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: The FLAGS trial. J. Clin. Oncol. 2010, 28, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA), Teysuno: EPAR Product Information. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001242/WC500104415.pdf (accessed on 26 July 2018).
- Ter Veer, E.; Mohammad, N.H.; Lodder, P.; Ngai, L.L.; Samaan, M.; van Oijen, M.G.; van Laarhoven, H.W. The efficacy and safety of S-1-based regimens in the first-line treatment of advanced gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2016, 19, 696–712. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Buyse, M.; Lichinitser, M.; Gorbunova, V.; Bodoky, G.; Douillard, J.Y.; Cascinu, S.; Heinemann, V.; Zaucha, R.; Carrato, A.; et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur. J. Cancer 2013, 49, 3616–3624. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Abramov, M.; Bondarenko, I.; Shparyk, Y.; Gorbunova, V.; Hontsa, A.; Otchenash, N.; Alsina, M.; Lazarev, S.; Feliu, J.; et al. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann. Oncol. 2017, 28, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, L.; Tian, L.; Cai, T.; Claggett, B.; Callegaro, A.; Dizier, B.; Spiessens, B.; Ulloa-Montoya, F.; Wei, L.J. A predictive enrichment procedure to identify potential responders to a new therapy for randomized, comparative controlled clinical studies. Biometrics 2016, 72, 877–887. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration, Guidance for Industry Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products, DRAFT GUIDANCE. 2012. Available online: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm332181.pdf (accessed on 26 July 2018).
- Takahari, D.; Mizusawa, J.; Koizumi, W.; Hyodo, I.; Boku, N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer 2017, 20, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Matsumoto, S.; Teramukai, S.; Ishiwata, R.; Nagai, Y.; Fukushima, M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007, 73, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hong, D.; Zhai, Y.; Shen, P. Meta-analysis of associations between neutrophil-to-lymphocyte ratio and prognosis of gastric cancer. World J. Surg. Oncol. 2015, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, H.G.; Engelhardt, E.G.; van Kleef, J.; Sprangers, M.A.G.; van Oijen, M.G.H.; Abu-Hanna, A.; Zwinderman, A.H.; Coupe, V.M.H.; van Laarhoven, H.W.M. Prediction models for patients with esophageal or gastric cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192310. [Google Scholar] [CrossRef] [PubMed]

| Covariate | Number of NA | Data Details |
|---|---|---|
| PS | 0 | PS 0 or PS 1 |
| Disease Type | 0 | (Local or 1 Distant Meta) or (≧2 Distant Meta) |
| Baseline site | 0 | Liver meta (+/−) |
| 0 | Lung meta (+/−) | |
| Tissue Type | 0 | Diffuse Type or Not Diffuse Type |
| Age | 0 | Continuous |
| Gender | 0 | Male or Female |
| Albumin | 69 | Continuous |
| Primary Region | 0 | Stomach (+/−) |
| N/L ratio | 24 | Continuous |
| Trial Label | 0 | Indicator of the FLAGS or DIGEST |
| Covariate | Number of NA | Data Details |
|---|---|---|
| PS | 0 | PS 0 or PS 1 |
| Disease Type | 1 | (Local or 1 Distant Meta) or (≧2 Distant Meta) |
| Baseline site | 0 | Liver meta (+/−) |
| 0 | Peritoneal meta (+/−) | |
| Tissue Type | 0 | Diffuse Type or Not Diffuse Type |
| Heart Rate | 24 | Continuous |
| Respiratory Rate | 95 | Continuous |
| Neutrophil | 1 | Continuous |
| Albumin | 44 | Continuous |
| Lymphocyte | 9 | Continuous |
| Primary Region | 0 | Stomach (+/−) |
| Hemoglobin | 1 | Continuous |
| Gender | 0 | Male or Female |
| Temperature | 34 | Continuous |
| FLAGS Trial | Investigator Assessment (Peritoneal Metastasis) | ||
| No | Yes | ||
| Central assessment(peritoneal metastasis) | No | 495 | 26 |
| Yes | 402 | 106 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, M.; Ajani, J.A.; Fang, X.; Pfeiffer, P.; Takeuchi, M.; van Laarhoven, H.W.M. Meta-Enrichment Analyses to Identify Advanced Gastric Cancer Patients Who Achieve a Higher Response to S-1/Cisplatin. Cancers 2019, 11, 871. https://doi.org/10.3390/cancers11060871
Takeuchi M, Ajani JA, Fang X, Pfeiffer P, Takeuchi M, van Laarhoven HWM. Meta-Enrichment Analyses to Identify Advanced Gastric Cancer Patients Who Achieve a Higher Response to S-1/Cisplatin. Cancers. 2019; 11(6):871. https://doi.org/10.3390/cancers11060871
Chicago/Turabian StyleTakeuchi, Madoka, Jaffer A. Ajani, Xuemin Fang, Per Pfeiffer, Masahiro Takeuchi, and Hanneke W. M. van Laarhoven. 2019. "Meta-Enrichment Analyses to Identify Advanced Gastric Cancer Patients Who Achieve a Higher Response to S-1/Cisplatin" Cancers 11, no. 6: 871. https://doi.org/10.3390/cancers11060871
APA StyleTakeuchi, M., Ajani, J. A., Fang, X., Pfeiffer, P., Takeuchi, M., & van Laarhoven, H. W. M. (2019). Meta-Enrichment Analyses to Identify Advanced Gastric Cancer Patients Who Achieve a Higher Response to S-1/Cisplatin. Cancers, 11(6), 871. https://doi.org/10.3390/cancers11060871






