Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation
Abstract
:1. Introduction
2. Results
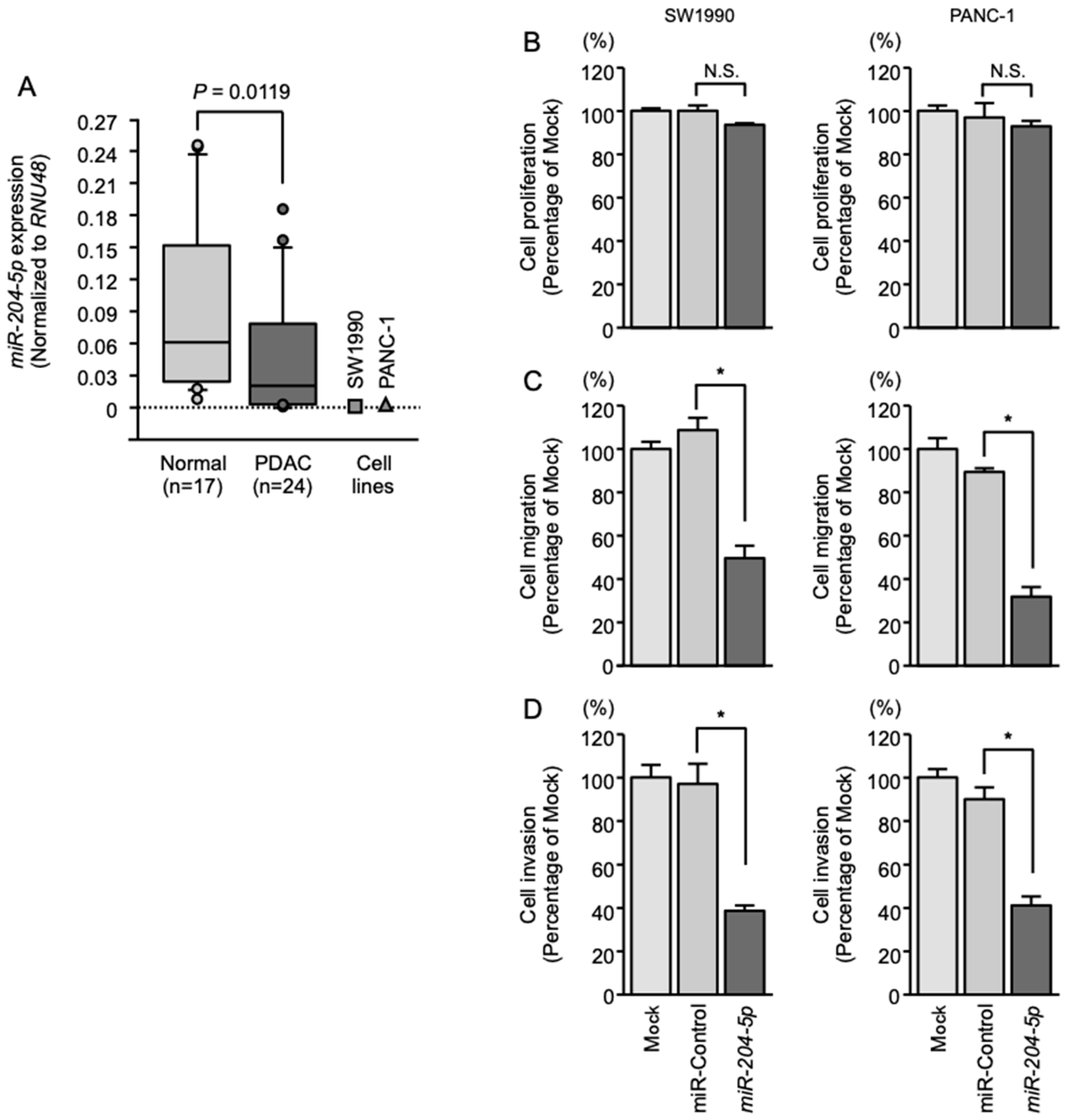
2.1. Expression of miR-204-5p in PDAC Specimens and Cell Lines
2.2. Effects of Overexpressing miR-204-5p on the Proliferation, Migration and Invasion of PDAC Cells
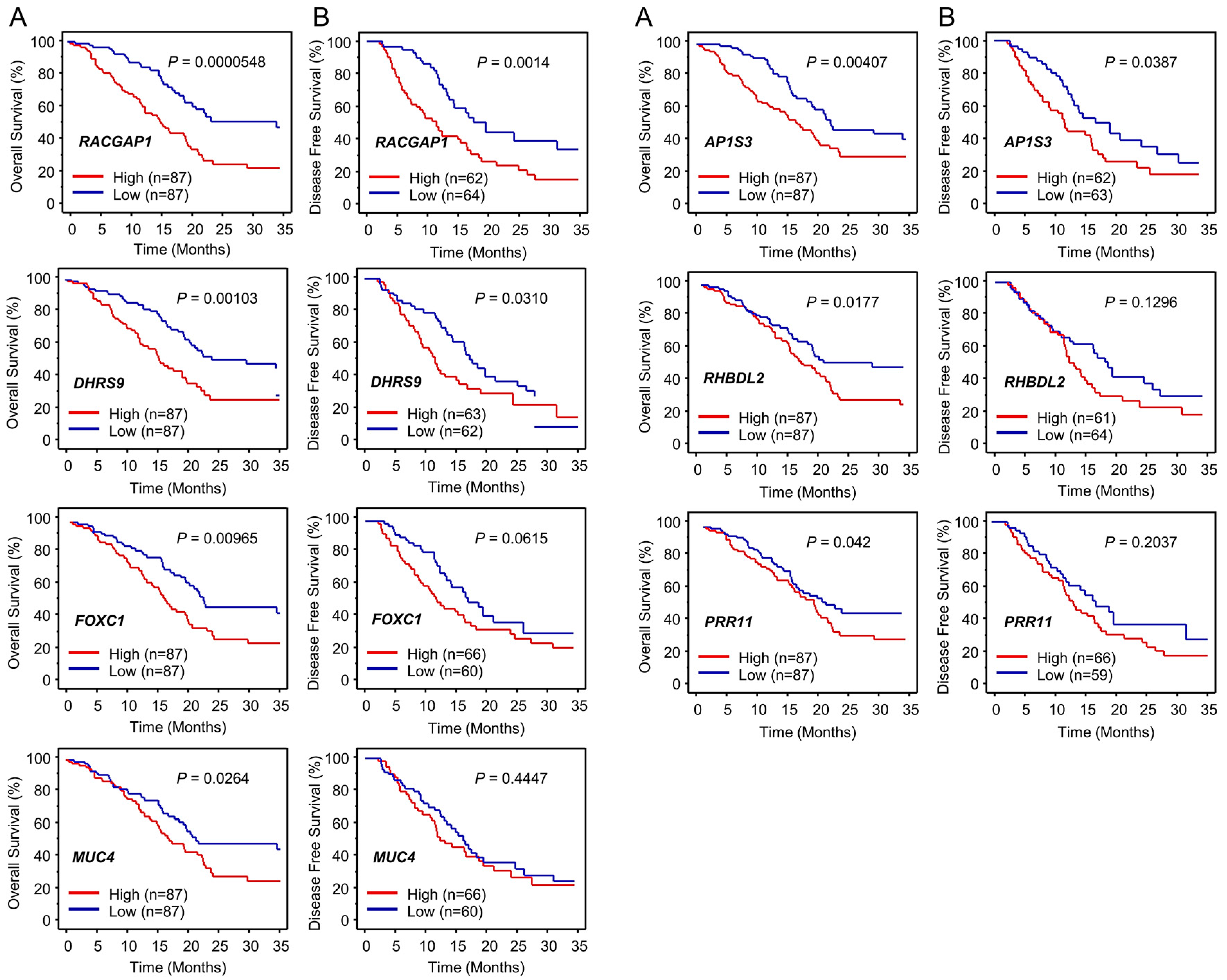
2.3. Identification of Putative Genes Regulated by miR-204-5p in PDAC Cells and Their Clinical Significance
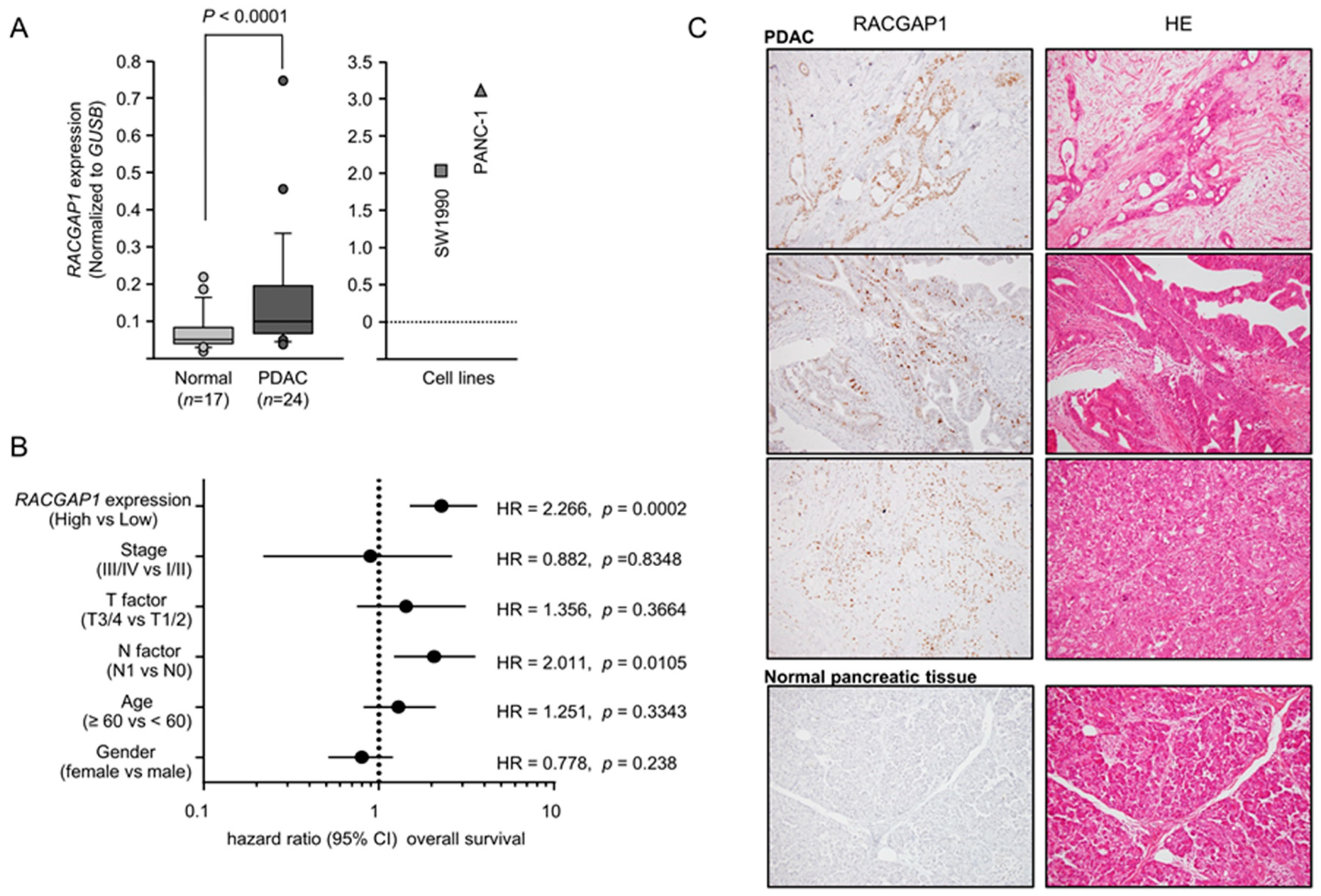
2.4. Expression of RACGAP1 in PDAC Clinical Specimens and Its Clinical Significance
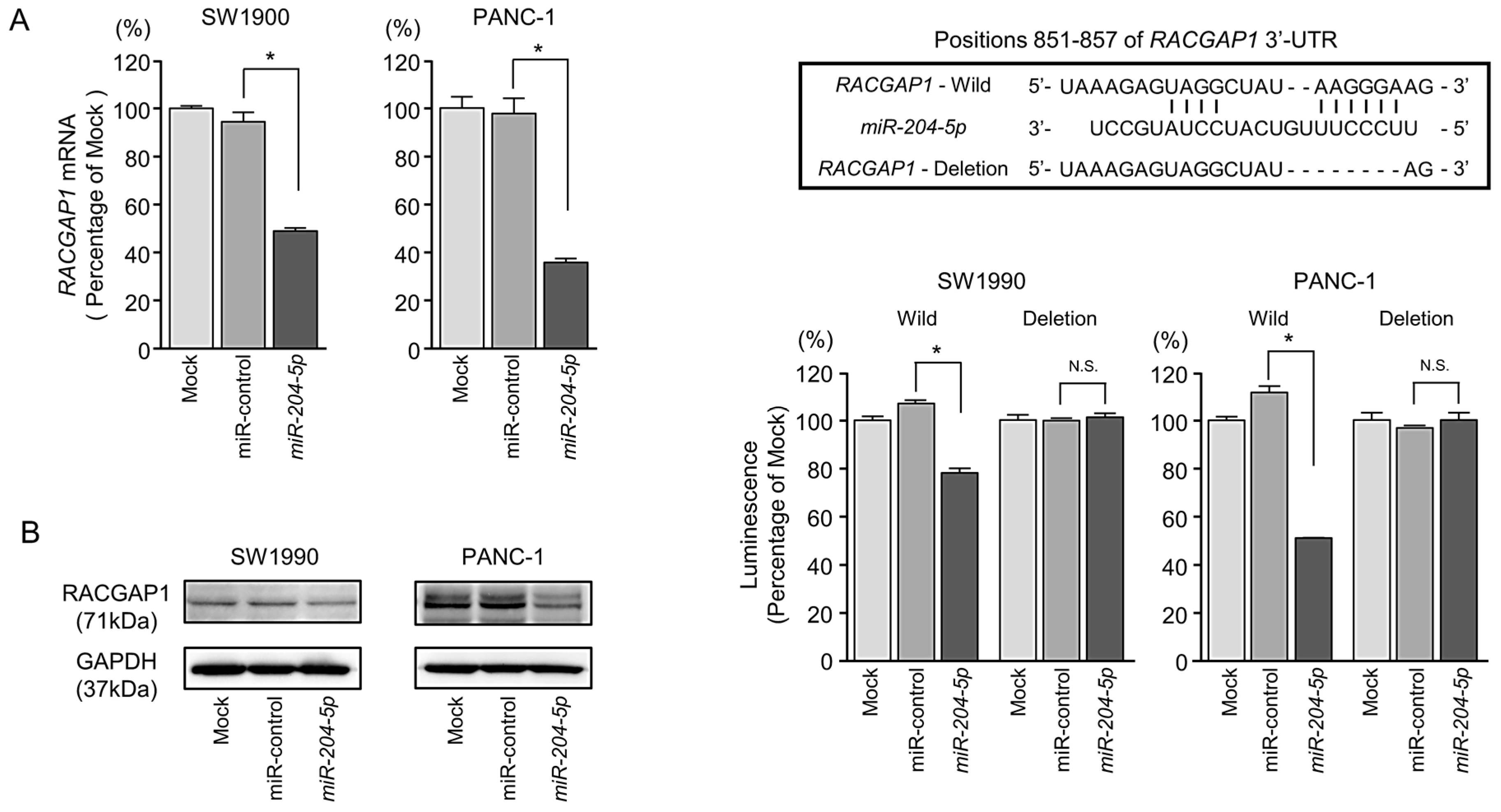
2.5. Direct Regulation of RACGAP1 by miR-204-5p in PDAC Cells
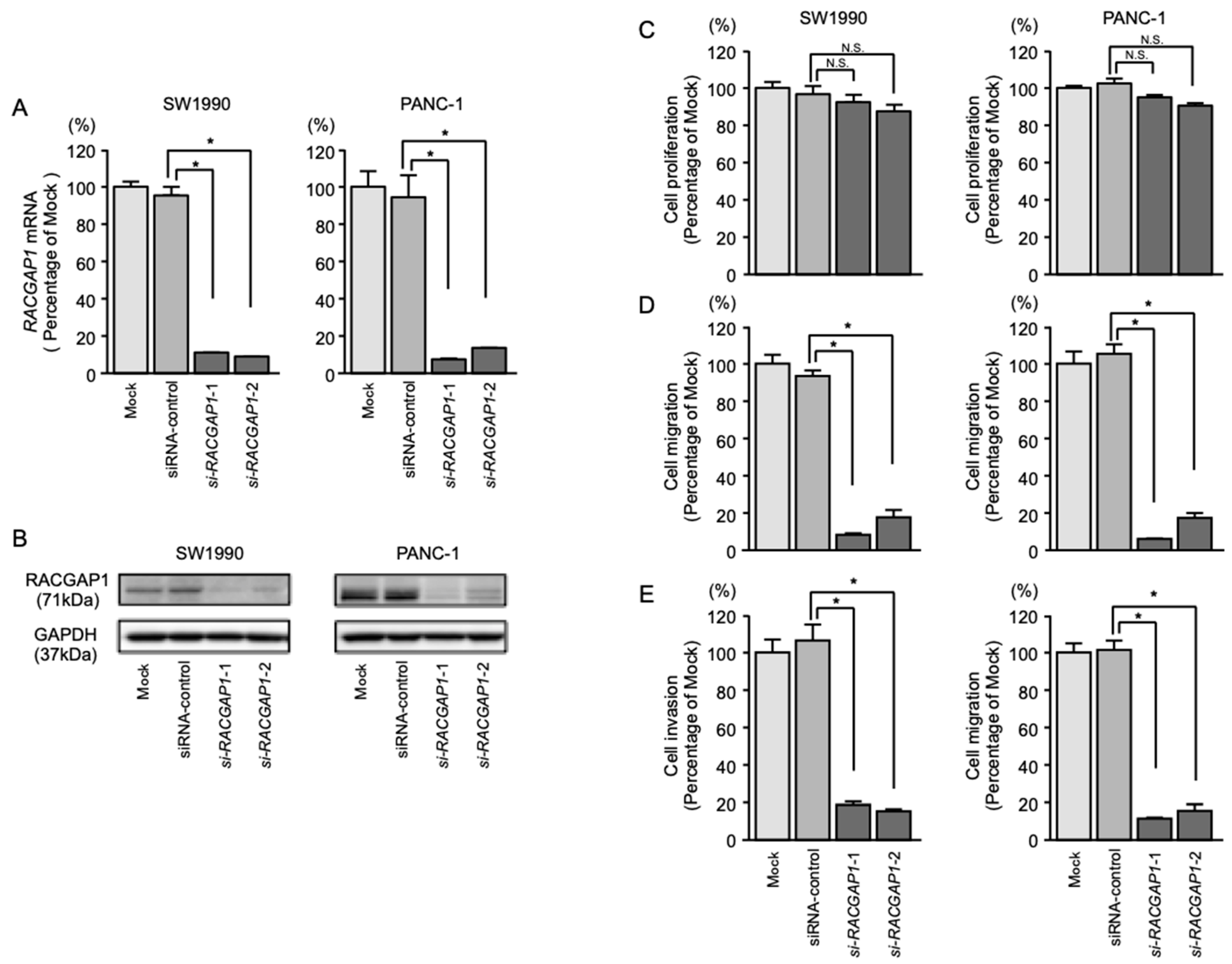
2.6. Effects of RACGAP1 Knockdown on the Proliferation, Migration and Invasion of PDAC Cells
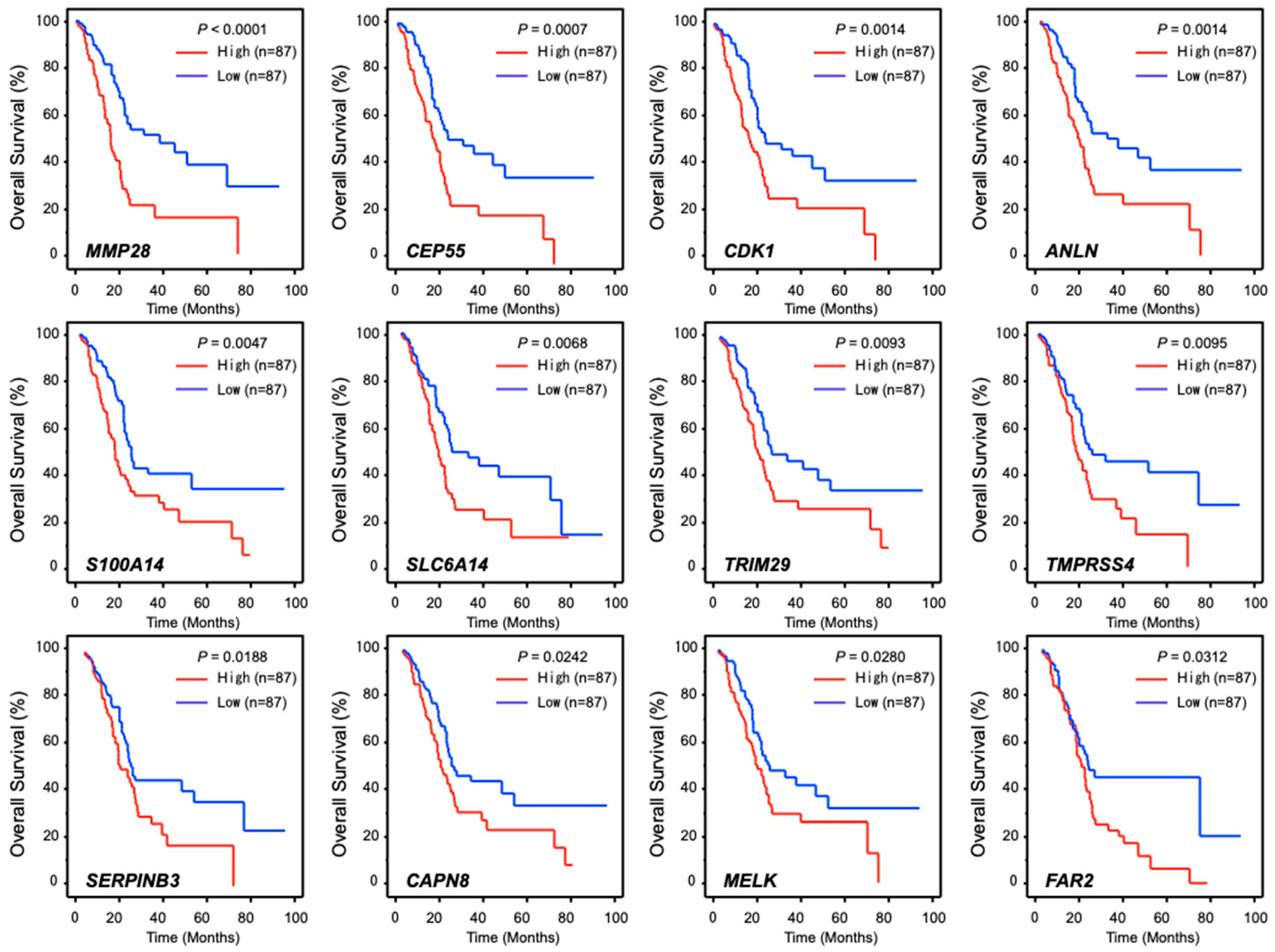
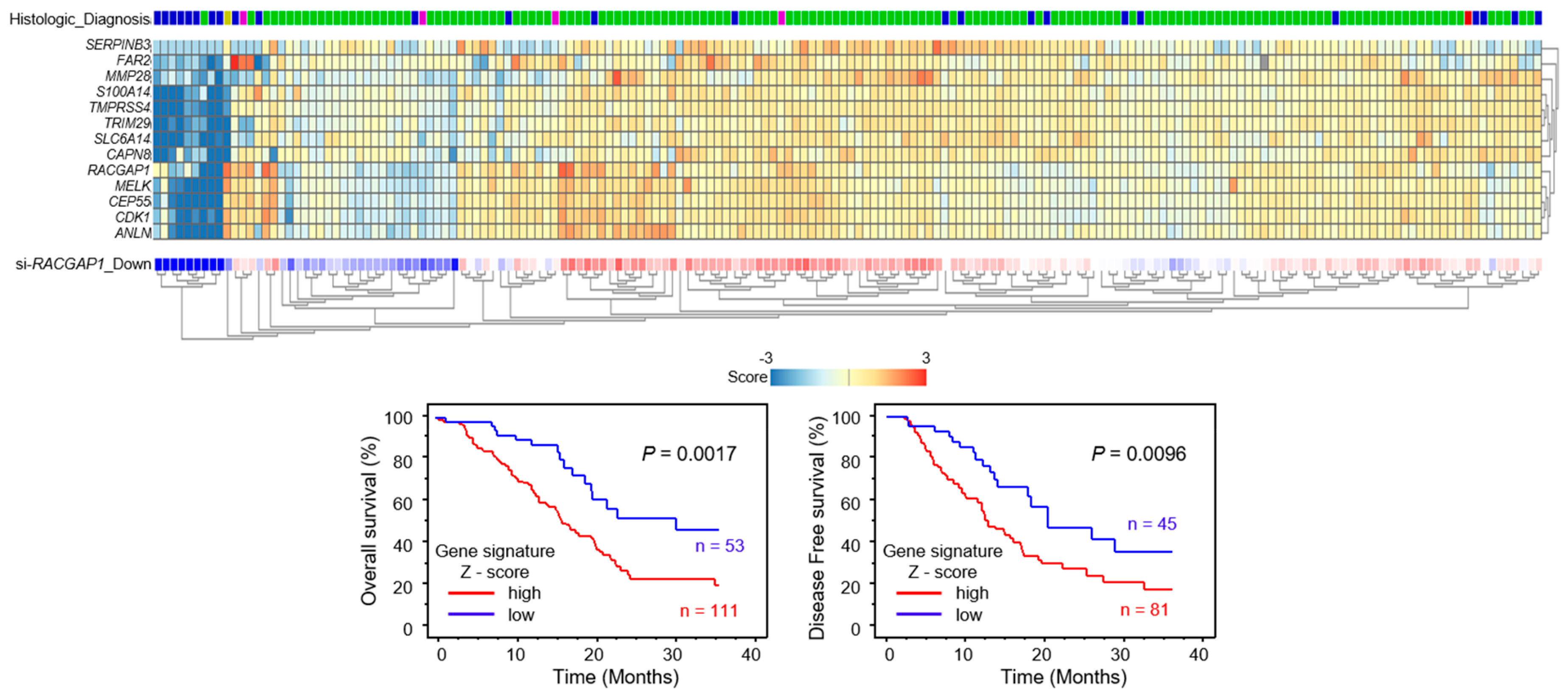
2.7. Identification of RACGAP1-Regulated Genes in PDAC Cells
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and PDAC Cell Lines
4.2. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.3. Transfection of Mature miRNAs and Small-Interfering RNAs (siRNAs)
4.4. Cell Proliferation, Migration and Invasion Assays
4.5. Identification of Genes Regulated by miR-204-5p in PDAC Cells
4.6. Clinical Database Analyses of PDAC
4.7. Plasmid Construction and Dual Luciferase Reporter Assay
4.8. Western Blotting and Immunohistochemistry
4.9. Downstream Genes Affected by RACGAP1 in PDAC Cells
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Chrystoja, C.C.; Diamandis, E.P.; Brand, R.; Ruckert, F.; Haun, R.; Molina, R. Pancreatic cancer. Clin. Chem. 2013, 59, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, Z.; Obazee, O.; Jia, J.; Childs, E.J.; Hoskins, J.; Figlioli, G.; Mocci, E.; Collins, I.; Chung, C.C.; et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget 2016, 7, 66328–66343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campa, D.; Rizzato, C.; Stolzenberg-Solomon, R.; Pacetti, P.; Vodicka, P.; Cleary, S.P.; Capurso, G.; Bueno-de-Mesquita, H.B.; Werner, J.; Gazouli, M.; et al. TERT gene harbors multiple variants associated with pancreatic cancer susceptibility. Int. J. Cancer 2015, 137, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Childs, E.J.; Mocci, E.; Campa, D.; Bracci, P.M.; Gallinger, S.; Goggins, M.; Li, D.; Neale, R.E.; Olson, S.H.; Scelo, G.; et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 2015, 47, 911–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.P.; Wolpin, B.M.; Risch, H.A.; Stolzenberg-Solomon, R.Z.; Mocci, E.; Zhang, M.; Canzian, F.; Childs, E.J.; Hoskins, J.W.; Jermusyk, A.; et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat. Commun. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z. MicroRNAs as oncogenes and tumor suppressors. N. Eng. J. Med. 2005, 353, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kurozumi, A.; Enokida, H.; Ichikawa, T.; Seki, N. Functional significance of aberrantly expressed microRNAs in prostate cancer. Int. J. Urol. 2015, 22, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshizuka, K.; Hanazawa, T.; Arai, T.; Okato, A.; Kikkawa, N.; Seki, N. Involvement of aberrantly expressed microRNAs in the pathogenesis of head and neck squamous cell carcinoma. Cancer Metastasis Rev. 2017, 36, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Kurahara, H.; Maemura, K.; Natsugoe, S. MicroRNA in pancreatic cancer. J. Hum. Genet. 2017, 62, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Seki, N.; Idichi, T.; Kurahara, H.; Osako, Y.; Koshizuka, K.; Arai, T.; Okato, A.; Kita, Y.; Arigami, T.; et al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: Anti-tumour functions of the microRNA-216 cluster. Oncotarget 2017, 8, 70097–70115. [Google Scholar] [CrossRef] [PubMed]
- Idichi, T.; Seki, N.; Kurahara, H.; Yonemori, K.; Osako, Y.; Arai, T.; Okato, A.; Kita, Y.; Arigami, T.; Mataki, Y.; et al. Regulation of actin-binding protein ANLN by antitumor miR-217 inhibits cancer cell aggressiveness in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 53180–53193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonemori, K.; Seki, N.; Kurahara, H.; Osako, Y.; Idichi, T.; Arai, T.; Koshizuka, K.; Kita, Y.; Maemura, K.; Natsugoe, S. ZFP36L2 promotes cancer cell aggressiveness and is regulated by antitumor microRNA-375 in pancreatic ductal adenocarcinoma. Cancer Sci. 2017, 108, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Idichi, T.; Seki, N.; Kurahara, H.; Fukuhisa, H.; Toda, H.; Shimonosono, M.; Okato, A.; Arai, T.; Kita, Y.; Mataki, Y.; et al. Molecular pathogenesis of pancreatic ductal adenocarcinoma: Impact of passenger strand of pre-miR-148a on gene regulation. Cancer Sci. 2018, 109, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Idichi, T.; Seki, N.; Kurahara, H.; Fukuhisa, H.; Toda, H.; Shimonosono, M.; Yamada, Y.; Arai, T.; Kita, Y.; Kijima, Y.; et al. Involvement of anti-tumor miR-124-3p and its targets in the pathogenesis of pancreatic ductal adenocarcinoma: Direct regulation of ITGA3 and ITGB1 by miR-124-3p. Oncotarget 2018, 9, 28849–28865. [Google Scholar] [CrossRef] [PubMed]
- Canu, V.; Sacconi, A.; Lorenzon, L.; Biagioni, F.; Lo Sardo, F.; Diodoro, M.G.; Muti, P.; Garofalo, A.; Strano, S.; D’Errico, A.; et al. MiR-204 down-regulation elicited perturbation of a gene target signature common to human cholangiocarcinoma and gastric cancer. Oncotarget 2017, 8, 29540–29557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Sangwan, V.; Banerjee, S.; Mackenzie, T.; Dudeja, V.; Li, X.; Wang, H.; Vickers, S.M.; Saluja, A.K. miR-204 mediated loss of myeloid cell leukemia-1 results in pancreatic cancer cell death. Mol. Cancer 2013, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, B.; Sun, X.; Lu, S.; Liu, Z.; Liu, Y.; Li, H.; Wang, L.; Wang, X.; Zhao, C. MiR-204 inhibits human NSCLC metastasis through suppression of NUAK1. Br. J. Cancer 2014, 111, 2316–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, H.; Kurozumi, S.; Kijima, Y.; Idichi, T.; Shinden, Y.; Yamada, Y.; Arai, T.; Maemura, K.; Fujii, T.; Horiguchi, J.; et al. Molecular pathogenesis of triple-negative breast cancer based on microRNA expression signatures: Antitumor miR-204-5p targets AP1S3. J. Hum. Genet. 2018, 63, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wu, Y.; He, X.; Zhang, C.; Zhu, M.; Chen, B.; Liu, Q.; Qu, X.; Li, W.; Wen, S.; et al. MicroRNA-204-5p inhibits invasion and metastasis of laryngeal squamous cell carcinoma by suppressing forkhead box C1. J. Cancer 2017, 8, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, L.; Xiao, S.J.; Liu, P.; Yi, S.J.; Zhang, X.L.; Ou-Yang, S.; Tan, S.K.; Lei, X. Forkhead box C1 induces epithelialmesenchymal transition and is a potential therapeutic target in nasopharyngeal carcinoma. Mol. Med. Rep. 2015, 12, 8003–8009. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Camacho, F.A.; Levin, C.I.; Flores, K.; Clift, A.; Galvez, A.; Terres, M.; Rivera, S.; Kolli, S.N.; Dodderer, J.; et al. FOXC1 plays a crucial role in the growth of pancreatic cancer. Oncogenesis 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Li, L.; Wang, L.; Yao, R.; Sun, Q.; Du, G. FOXC1 is associated with estrogen receptor alpha and affects sensitivity of tamoxifen treatment in breast cancer. Cancer Med. 2017, 6, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nature Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhi, X.; Zhu, Y.; Zhang, Q.; Wang, W.; Li, Z.; Tang, J.; Wang, J.; Wei, S.; Li, B.; et al. MUC4-promoted neural invasion is mediated by the axon guidance factor Netrin-1 in PDAC. Oncotarget 2015, 6, 33805–33822. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Choi, A.H.; Deng, Z.; Yang, Y.; Zhao, J.; Wang, Y.; Hardwidge, P.R.; Zhu, G. Cell membrane-anchored MUC4 promotes tumorigenicity in epithelial carcinomas. Oncotarget 2017, 8, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Bernards, A.; Settleman, J. GAP control: Regulating the regulators of small GTPases. Trends Cell Biol. 2004, 14, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Ooi, L.L.; Hui, K.M. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 6040–6051. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H.; Toiyama, Y.; Saigusa, S.; Kawamura, M.; Kawamoto, A.; Okugawa, Y.; Hiro, J.; Tanaka, K.; Inoue, Y.; Mohri, Y.; et al. RacGAP1 expression, increasing tumor malignant potential, as a predictive biomarker for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 2015, 36, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigusa, S.; Tanaka, K.; Mohri, Y.; Ohi, M.; Shimura, T.; Kitajima, T.; Kondo, S.; Okugawa, Y.; Toiyama, Y.; Inoue, Y.; et al. Clinical significance of RacGAP1 expression at the invasive front of gastric cancer. Gastric Cancer. 2015, 18, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lin, M.; Brouwer-Visser, J.; Heim, J.; Smotkin, D.; Hebert, T.; Gunter, M.J.; Goldberg, G.L.; Zheng, D.; Huang, G.S. RNA-seq Identification of RACGAP1 as a Metastatic Driver in Uterine Carcinosarcoma. Clin. Cancer Res. 2016, 22, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, W.; Liu, Y.; Yong, M.; Yang, Y.; Zhou, H. Rac GTPase activating protein 1 promotes oncogenic progression of epithelial ovarian cancer. Cancer Sci. 2018, 109, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hazar-Rethinam, M.; de Long, L.M.; Gannon, O.M.; Boros, S.; Vargas, A.C.; Dzienis, M.; Mukhopadhyay, P.; Saenz-Ponce, N.; Dantzic, D.D.; Simpson, F.; et al. RacGAP1 is a novel downstream effector of e2f7-dependent resistance to doxorubicin and is prognostic for overall survival in squamous cell carcinoma. Mol. Cancer Therap. 2015, 14, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, M.K.; Ndebele, K.; Bucur, O.; Yee, E.U.; Otu, H.H.; Plati, J.; Bullock, A.; Gu, X.; Castan, E.; Zhang, P.; et al. Meta-analysis of transcriptome data identifies a novel 5-gene pancreatic adenocarcinoma classifier. Oncotarget 2016, 7, 23263–23281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Liang, J.; Bai, L. MicroRNA-449a functions as a tumor suppressor in pancreatic cancer by the epigenetic regulation of ATDC expression. Biomed. Pharmacother. 2018, 103, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kojima, S.; Yamada, Y.; Sugawara, S.; Kato, M.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Pirin: A potential novel therapeutic target for castration-resistant prostate cancer regulated by miR-455-5p. Mol. Oncol. 2018, 13, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Arai, T.; Kojima, S.; Sugawara, S.; Kato, M.; Okato, A.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 2018, 109, 2919–2936. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Arai, T.; Sugawara, S.; Okato, A.; Kato, M.; Kojima, S.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Impact of novel oncogenic pathways regulated by antitumor miR-451a in renal cell carcinoma. Cancer Sci. 2018, 109, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sugawara, S.; Arai, T.; Kojima, S.; Kato, M.; Okato, A.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Molecular pathogenesis of renal cell carcinoma: Impact of the anti-tumor miR-29 family on gene regulation. Int. J. Urol. 2018, 25, 953–965. [Google Scholar] [CrossRef] [PubMed]
| Pancreatic Ductal Adenocarcinoma (PDAC) | Factors | Number | (%) |
|---|---|---|---|
| Total number | 24 | ||
| Average age(range), years | 67.0 (42–79) | ||
| Gender | Male | 12 | (50.0) |
| Female | 12 | (50.0) | |
| T category | pT1 | 1 | (4.2) |
| pT2 | 0 | (0) | |
| pT3 | 23 | (95.8) | |
| pT4 | 0 | (0) | |
| N category | 0 | 8 | (33.3) |
| 1 | 16 | (66.7) | |
| M category | 0 | 23 | (95.8) |
| 1 | 1 | (4.2) | |
| Lymphatic invasion | 0 | 1 | (4.2) |
| 1 | 11 | (45.8) | |
| 2 | 9 | (37.5) | |
| 3 | 3 | (12.5) | |
| Vascular invasion | 0 | 1 | (4.2) |
| 1 | 10 | (41.7) | |
| 2 | 11 | (45.8) | |
| 3 | 2 | (8.3) | |
| Neoadjuvant Chemotherapy | (−) | 12 | (50.0) |
| (+) | 12 | (50.0) | |
| Adjuvant Chemotherapy | (−) | 7 | (29.2) |
| (+) | 17 | (70.8) | |
| Recurrence | (−) | 7 | (29.2) |
| (+) | 17 | (70.8) |
| Normal Pancreatic Tissue | Factors | Number | (%) |
|---|---|---|---|
| Total number | 17 | ||
| Average age (range), years | 64.8 (42–85) | ||
| Gender | Male | 6 | (35.3) |
| Female | 11 | (64.7) |
| Entrez Gene ID | Gene Symbol | Gene Name | Target Sites | GEO | Array (SW1990) | OncoLnc OS_p-Value PDAC | |
|---|---|---|---|---|---|---|---|
| Conserved Sites | Poorly Sites | FC (log) | Mock vs. miR-204-5p | ||||
| 29127 | RACGAP1 | Rac GTPase activating protein 1 | 0 | 1 | 1.34 | −1.016 | 0.00005 |
| 10170 | DHRS9 | dehydrogenase/reductase (SDR family) member 9 | 0 | 2 | 1.57 | −1.078 | 0.00103 |
| 130340 | AP1S3 | adaptor-related protein complex 1, sigma 3 subunit | 0 | 2 | 1.17 | −3.004 | 0.00407 |
| 2296 | FOXC1 | forkhead box C1 | 1 | 1 | 1.01 | −1.059 | 0.00965 |
| 54933 | RHBDL2 | rhomboid, veinlet-like 2 (Drosophila) | 0 | 1 | 1.18 | −1.061 | 0.01770 |
| 4585 | MUC4 | mucin 4, cell surface associated | 0 | 1 | 1.42 | −1.569 | 0.02640 |
| 55771 | PRR11 | proline rich 11 | 1 | 0 | 1.42 | −1.406 | 0.04200 |
| 4680 | CEACAM6 | carcinoembryonic antigen-related cell adhesion molecule 6 (non-specific cross reacting antigen) | 0 | 1 | 3.50 | −1.260 | 0.07160 |
| 6505 | SLC1A1 | solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 | 0 | 2 | 1.52 | −1.397 | 0.08320 |
| 2335 | FN1 | fibronectin 1 | 0 | 1 | 3.73 | −1.023 | 0.09500 |
| 3397 | ID1 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | 0 | 1 | 1.29 | −1.384 | 0.15300 |
| 55808 | ST6GALNAC1 | ST6 (α-N-acetyl-neuraminyl-2,3-β-galactosyl-1,3)-N-acetylgalactosaminide alpha-2,6-sialyltransferase 1 | 0 | 1 | 1.36 | −1.367 | 0.15300 |
| 54210 | TREM1 | triggering receptor expressed on myeloid cells 1 | 0 | 1 | 1.55 | −1.067 | 0.15700 |
| 8905 | AP1S2 | adaptor-related protein complex 1, sigma 2 subunit | 2 | 0 | 1.04 | −1.806 | 0.17700 |
| 147495 | APCDD1 | adenomatosis polyposis coli down-regulated 1 | 0 | 1 | 1.18 | −1.276 | 0.21200 |
| 4837 | NNMT | nicotinamide N-methyltransferase | 0 | 1 | 2.69 | −1.060 | 0.21900 |
| 7851 | MALL | mal, T-cell differentiation protein-like | 1 | 1 | 1.44 | −1.252 | 0.22600 |
| 493 | ATP2B4 | ATPase, Ca++ transporting, plasma membrane 4 | 0 | 1 | 1.35 | −1.149 | 0.23600 |
| 1295 | COL8A1 | collagen, type VIII, α 1 | 0 | 1 | 4.62 | −1.131 | 0.36400 |
| 219699 | UNC5B | unc-5 homolog B (C. elegans) | 1 | 1 | 1.42 | −1.628 | 0.59600 |
| 2192 | FBLN1 | fibulin 1 | 0 | 1 | 1.62 | −1.262 | 0.65300 |
| 5159 | PDGFRB | Platelet-derived growth factor receptor, beta polypeptide | 0 | 2 | 1.80 | −2.235 | 0.77500 |
| 2182 | ACSL4 | acyl-CoA synthetase long-chain family member 4 | 1 | 2 | 1.05 | −1.570 | 0.79200 |
| 140885 | SIRPA | signal-regulatory protein alpha | 0 | 1 | 1.20 | −1.194 | 0.80000 |
| 1809 | DPYSL3 | dihydropyrimidinase-like 3 | 1 | 1 | 2.07 | −1.185 | 0.99600 |
| Entrez GeneID | Gene Symbol | GeneName | miR-204-5p Conserved Sites Total | miR-204-5p Poorly Conserved Sites Total | GEO FC (log) | Array(SW1990) Mock vs. si-RACGAP1 | OncoLnc OS_p-Value PAAD |
|---|---|---|---|---|---|---|---|
| 79148 | MMP28 | matrix metallopeptidase 28 | - | - | 1.44652 | −1.30890 | 1.45E-05 |
| 29127 | RACGAP1 | Rac GTPase activating protein 1 | 0 | 1 | 1.34429 | −3.04118 | 0.0000548 |
| 55165 | CEP55 | centrosomal protein 55 kDa | - | - | 1.09192 | −1.05821 | 0.000736 |
| 983 | CDK1 | cyclin-dependent kinase 1 | - | - | 1.41425 | −1.03595 | 0.00137 |
| 54443 | ANLN | anillin, actin binding protein | - | - | 1.72921 | −1.19268 | 0.00142 |
| 57402 | S100A14 | S100 calcium binding protein A14 | - | - | 1.06798 | −1.19808 | 0.00469 |
| 11254 | SLC6A14 | solute carrier family 6 (amino acid transporter), member 14 | 0 | 1 | 3.01708 | −1.20549 | 0.00678 |
| 23650 | TRIM29 | tripartite motif containing 29 | - | - | 2.00490 | −1.23048 | 0.00934 |
| 56649 | TMPRSS4 | transmembrane protease, serine 4 | - | - | 1.96115 | −1.27289 | 0.00954 |
| 6317 | SERPINB3 | serpin peptidase inhibitor, clade B (ovalbumin), member 3 | - | - | 1.74282 | −1.15475 | 0.0188 |
| 388743 | CAPN8 | calpain 8 | - | - | 1.92729 | −2.40223 | 0.0242 |
| 9833 | MELK | maternal embryonic leucine zipper kinase | 0 | 1 | 1.21153 | −1.04052 | 0.028 |
| 55711 | FAR2 | fatty acyl CoA reductase 2 | - | - | 1.04894 | −1.13250 | 0.0312 |
| 1893 | ECM1 | extracellular matrix protein 1 | - | - | 1.14510 | −1.03234 | 0.0531 |
| 11178 | LZTS1 | leucine zipper, putative tumor suppressor 1 | - | - | 1.30061 | −1.64372 | 0.0591 |
| 6696 | SPP1 | secreted phosphoprotein 1 | - | - | 2.67411 | −1.79208 | 0.0639 |
| 5349 | FXYD3 | FXYD domain containing ion transport regulator 3 | - | - | 1.80037 | −1.46428 | 0.0677 |
| 4680 | CEACAM6 | carcinoembryonic antigen-related cell adhesion molecule 6 (non-specific cross reacting antigen) | 0 | 1 | 3.49703 | −1.04848 | 0.0716 |
| 80736 | SLC44A4 | solute carrier family 44, member 4 | - | - | 1.84270 | −1.14495 | 0.0761 |
| 80856 | KIAA1715 | KIAA1715 | 0 | 2 | 1.05264 | −1.70461 | 0.0891 |
| 412 | STS | steroid sulfatase (microsomal), isozyme S | - | - | 1.32435 | −2.62844 | 0.0996 |
| 1087 | CEACAM7 | carcinoembryonic antigen-related cell adhesion molecule 7 | - | - | 1.89107 | −1.11293 | 0.105 |
| 29969 | MDFIC | MyoD family inhibitor domain containing | 0 | 1 | 1.20813 | −1.20299 | 0.11 |
| 23596 | OPN3 | opsin 3 | - | - | 1.28539 | −1.53133 | 0.122 |
| 56241 | SUSD2 | sushi domain containing 2 | - | - | 1.30384 | −1.26476 | 0.123 |
| 55808 | ST6GALNAC1 | ST6 (alpha-N-acetyl-neuraminyl-2,3-β-galactosyl-1,3)-N-acetylgalactosaminide α-2,6-sialyltransferase 1 | 0 | 1 | 1.35766 | −1.55278 | 0.153 |
| 90459 | ERI1 | exoribonuclease 1 | - | - | 1.08766 | −1.07601 | 0.158 |
| 1999 | ELF3 | E74-like factor 3 (ETS domain transcription factor, epithelial-specific) | - | - | 1.18538 | −1.05779 | 0.174 |
| 121457 | IKBIP | IKBKB interacting protein | - | - | 1.10964 | −1.06344 | 0.185 |
| 50810 | HDGFRP3 | hepatoma-derived growth factor, related protein 3 | - | - | 1.37815 | −1.69105 | 0.187 |
| 1048 | CEACAM5 | carcinoembryonic antigen-related cell adhesion molecule 5 | - | - | 2.76693 | −1.96132 | 0.188 |
| 147495 | APCDD1 | adenomatosis polyposis coli down-regulated 1 | 0 | 1 | 1.17965 | −1.33923 | 0.212 |
| 200958 | MUC20 | mucin 20, cell surface associated | - | - | 1.50494 | −1.28329 | 0.232 |
| 54843 | SYTL2 | synaptotagmin-like 2 | - | - | 1.90048 | −1.17307 | 0.242 |
| 7031 | TFF1 | trefoil factor 1 | - | - | 2.40095 | −1.13688 | 0.242 |
| 1847 | DUSP5 | dual specificity phosphatase 5 | - | - | 1.47556 | −1.77597 | 0.269 |
| 85477 | SCIN | scinderin | - | - | 1.14779 | −1.62677 | 0.285 |
| 10000 | AKT3 | v-akt murine thymoma viral oncogene homolog 3 | - | - | 1.02342 | −1.07780 | 0.317 |
| 89932 | PAPLN | papilin, proteoglycan-like sulfated glycoprotein | 0 | 2 | 1.81417 | −1.08233 | 0.332 |
| 51056 | LAP3 | leucine aminopeptidase 3 | - | - | 1.06387 | −1.23137 | 0.343 |
| 4688 | NCF2 | neutrophil cytosolic factor 2 | - | - | 2.00063 | −1.14183 | 0.422 |
| 22795 | NID2 | nidogen 2 (osteonidogen) | - | - | 1.78329 | −1.09923 | 0.425 |
| 8706 | B3GALNT1 | β-1,3-N-acetyl-galactosaminyl-transferase 1 (globoside blood group) | - | - | 1.06403 | −1.07457 | 0.474 |
| 10551 | AGR2 | anterior gradient 2 | - | - | 2.04850 | −1.20759 | 0.491 |
| 1009 | CDH11 | cadherin 11, type 2, OB-cadherin (osteoblast) | 1 | 0 | 3.64065 | −1.32668 | 0.526 |
| 340547 | VSIG1 | V-set and immunoglobulin domain containing 1 | - | - | 1.78237 | −1.44981 | 0.54 |
| 219699 | UNC5B | unc-5 homolog B (C. elegans) | 1 | 1 | 1.42242 | −1.48944 | 0.596 |
| 154141 | MBOAT1 | membrane bound O-acyltransferase domain containing 1 | - | - | 1.03451 | −1.03993 | 0.615 |
| 3455 | IFNAR2 | interferon (α, β and ω) receptor 2 | 0 | 3 | 1.07229 | −1.11007 | 0.618 |
| 2357 | FPR1 | formyl peptide receptor 1 | 0 | 2 | 1.09684 | −1.92665 | 0.629 |
| 29887 | SNX10 | sorting nexin 10 | - | - | 1.20341 | −1.63818 | 0.64 |
| 91607 | SLFN11 | Schlafen family member 11 | - | - | 1.37401 | −1.23024 | 0.661 |
| 79071 | ELOVL6 | ELOVL fatty acid elongase 6 | 1 | 2 | 1.48750 | −1.18773 | 0.727 |
| 3075 | CFH | complement factor H | - | - | 1.64335 | −1.25960 | 0.732 |
| 5159 | PDGFRB | platelet-derived growth factor receptor, β polypeptide | 0 | 2 | 1.79906 | −3.93598 | 0.775 |
| 10855 | HPSE | heparanase | - | - | 1.10037 | −1.25724 | 0.783 |
| 2877 | GPX2 | glutathione peroxidase 2 (gastrointestinal) | - | - | 2.06843 | −1.24329 | 0.84 |
| 634 | CEACAM1 | carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) | - | - | 1.41681 | −1.08376 | 0.843 |
| 54749 | EPDR1 | ependymin related 1 | - | - | 1.00831 | −1.25356 | 0.86 |
| 2124 | EVI2B | ecotropic viral integration site 2B | - | - | 1.54469 | −1.32368 | 0.939 |
| 3310 | HSPA6 | heat shock 70kDa protein 6 (HSP70B’) | - | - | 1.34685 | −1.10270 | 0.956 |
| 6453 | ITSN1 | intersectin 1 (SH3 domain protein) | - | - | 1.21741 | −1.04096 | 0.982 |
| 30001 | ERO1L | ERO1-like (S. cerevisiae) | 0 | 1 | 1.05476 | −1.33509 | No data |
| 100499177 | THAP9-AS1 | THAP9 antisense RNA 1 | - | - | 1.02990 | −1.06954 | No data |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.; Idichi, T.; Seki, N.; Wada, M.; Yamada, Y.; Fukuhisa, H.; Toda, H.; Kita, Y.; Kawasaki, Y.; Tanoue, K.; et al. Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation. Cancers 2019, 11, 327. https://doi.org/10.3390/cancers11030327
Khalid M, Idichi T, Seki N, Wada M, Yamada Y, Fukuhisa H, Toda H, Kita Y, Kawasaki Y, Tanoue K, et al. Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation. Cancers. 2019; 11(3):327. https://doi.org/10.3390/cancers11030327
Chicago/Turabian StyleKhalid, Muhammad, Tetsuya Idichi, Naohiko Seki, Masumi Wada, Yasutaka Yamada, Haruhi Fukuhisa, Hiroko Toda, Yoshiaki Kita, Yota Kawasaki, Kiyonori Tanoue, and et al. 2019. "Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation" Cancers 11, no. 3: 327. https://doi.org/10.3390/cancers11030327





