Lung Tumorigenesis Alters the Expression of Slit2-exon15 Splicing Variants in Tumor Microenvironment
Abstract
:1. Introduction
2. Results
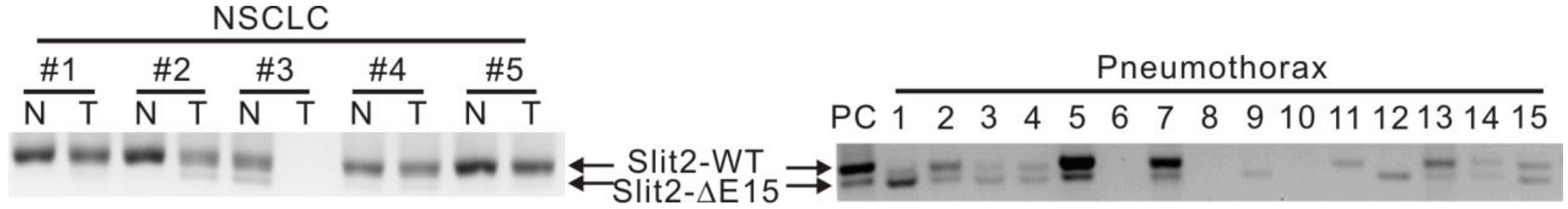
2.1. Expressions of Slit2-Exon15 Splicing Variants in Lung Cancer and Non-Lung Cancer Patients
2.2. Expressions of Slit2 Isoforms in Normal Lungs and Metastasized Lungs in Nude Mice Injected with Lung Cancer Cells via Tail Vein
2.3. Expressions of Slit2-Exon15 Isoforms in Lung Inflammation Induced by Lipopolysaccharide (LPS)
2.4. Expressions of Slit2-Exon15 Isoforms in kRasG12D-Induced Lung Cancer
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Intravenous Injection of Lung Cancer Cells
4.3. Lung Inflammation Induced by Intranasal Administration of Lipopolysaccharide (LPS)
4.4. Lung Tumor Formation in kRasG12D Mice
4.5. RNA Extraction and Real-Time PCR
4.6. Histology
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96, 795–806. [Google Scholar] [CrossRef]
- Dickson, B.J.; Gilestro, G.F. Regulation of commissural axon pathfinding by slit and its Robo receptors. Ann. Rev. Cell Dev. Biol. 2006, 22, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Challa, A.K.; Beattie, C.E.; Seeger, M.A. Identification and characterization of roundabout orthologs in zebrafish. Mech. Dev. 2001, 101, 249–253. [Google Scholar] [CrossRef]
- Huminiecki, L.; Gorn, M.; Suchting, S.; Poulsom, R.; Bicknell, R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 2002, 79, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ray, R.; Chien, C.B. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell migration. Dev. Dyn. 2001, 221, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; van Eerde, A.M.; Fan, X.; Quintero-Rivera, F.; Kulkarni, S.; Ferguson, H.; Kim, H.G.; Fan, Y.; Xi, Q.; Li, Q.G.; et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am. J. Hum. Genet. 2007, 80, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Moran, A.; Samara, Y.; Moreno, M.; Compton, J.E.; Harburg, G.; Strickland, P.; Hinck, L. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev. Cell 2011, 20, 827–840. [Google Scholar] [CrossRef]
- Medioni, C.; Bertrand, N.; Mesbah, K.; Hudry, B.; Dupays, L.; Wolstein, O.; Washkowitz, A.J.; Papaioannou, V.E.; Mohun, T.J.; Harvey, R.P.; et al. Expression of Slit and Robo genes in the developing mouse heart. Dev. Dyn. 2010, 239, 3303–3311. [Google Scholar] [CrossRef] [Green Version]
- Mommersteeg, M.T.; Andrews, W.D.; Ypsilanti, A.R.; Zelina, P.; Yeh, M.L.; Norden, J.; Kispert, A.; Chedotal, A.; Christoffels, V.M.; Parnavelas, J.G. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ. Res. 2013, 112, 465–475. [Google Scholar] [CrossRef]
- Piper, M.; Georgas, K.; Yamada, T.; Little, M. Expression of the vertebrate Slit gene family and their putative receptors, the Robo genes, in the developing murine kidney. Mech. Dev. 2000, 94, 213–217. [Google Scholar] [CrossRef]
- Dallol, A.; Da Silva, N.F.; Viacava, P.; Minna, J.D.; Bieche, I.; Maher, E.R.; Latif, F. SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002, 62, 5874–5880. [Google Scholar] [PubMed]
- Dallol, A.; Krex, D.; Hesson, L.; Eng, C.; Maher, E.R.; Latif, F. Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene 2003, 22, 4611–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallol, A.; Morton, D.; Maher, E.R.; Latif, F. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Res. 2003, 63, 1054–1058. [Google Scholar] [PubMed]
- Wang, B.; Xiao, Y.; Ding, B.B.; Zhang, N.; Yuan, X.; Gui, L.; Qian, K.X.; Duan, S.; Chen, Z.; Rao, Y.; et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003, 4, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Yang, Z.; Liu, W.; Liu, B.; Xu, Z.; Zhang, Z. Knockdown of Slit2 promotes growth and motility in gastric cancer cells via activation of AKT/beta-catenin. Oncol. Rep. 2014, 31, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.L.; Gonda, Y.; Mommersteeg, M.T.; Barber, M.; Ypsilanti, A.R.; Hanashima, C.; Parnavelas, J.G.; Andrews, W.D. Robo1 modulates proliferation and neurogenesis in the developing neocortex. J. Neurosci. 2014, 34, 5717–5731. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, X.; Kuzontkoski, P.M.; Jiang, S.; Zhu, W.; Li, D.Y.; Groopman, J.E. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun. Signal. 2014, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.J.; Yang, Y.; Yang, G.K.; Wan, J.; Cui, D.L.; Ma, Z.H.; Du, L.J.; Zhang, G.M. Slit2 suppresses endothelial cell proliferation and migration by inhibiting the VEGF-Notch signaling pathway. Mol. Med. Rep. 2017, 15, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Q.; Zhou, D.L.; Lei, Y.; Zheng, L.; Chen, S.X.; Gou, H.J.; Gu, Q.L.; He, X.D.; Lan, T.; Qi, C.L.; et al. Slit2/Robo1 signaling promotes intestinal tumorigenesis through Src-mediated activation of the Wnt/beta-catenin pathway. Oncotarget 2015, 6, 3123–3135. [Google Scholar] [CrossRef]
- Zhao, S.J.; Shen, Y.F.; Li, Q.; He, Y.J.; Zhang, Y.K.; Hu, L.P.; Jiang, Y.Q.; Xu, N.W.; Wang, Y.J.; Li, J.; et al. SLIT2/ROBO1 axis contributes to the Warburg effect in osteosarcoma through activation of SRC/ERK/c-MYC/PFKFB2 pathway. Cell Death Dis. 2018, 9, 390. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Yang, C.H.; Sheu, G.T.; Huang, C.Y.; Wu, Y.C.; Chuang, S.M.; Fann, M.J.; Chang, H.; Lee, H.; Chang, J.T. A novel exon 15-deleted, splicing variant of Slit2 shows potential for growth inhibition in addition to invasion inhibition in lung cancer. Cancer 2011, 117, 3404–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.C.; Chen, P.N.; Wang, S.Y.; Liao, C.Y.; Lin, Y.Y.; Sun, S.R.; Chiu, C.L.; Hsieh, Y.S.; Shieh, J.C.; Chang, J.T. The differential roles of Slit2-exon 15 splicing variants in angiogenesis and HUVEC permeability. Angiogenesis 2015, 18, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Feng, L.; Park, H.T.; Havlioglu, N.; Wen, L.; Tang, H.; Bacon, K.B.; Jiang, Z.; Zhang, X.; Rao, Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 2001, 410, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Qamri, Z.; Wu, J.; Ganju, R.K. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J. Leukoc. Biol. 2007, 82, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Zu, G.; Xie, Y.; Tang, H.; Johnson, M.; Xu, X.; Kevil, C.; Xiong, W.C.; Elmets, C.; Rao, Y.; et al. Neuronal repellent Slit2 inhibits dendritic cell migration and the development of immune responses. J. Immunol. 2003, 171, 6519–6526. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Garcia, G.E.; Li, P.; Parra, G.; Wilson, C.B.; Rao, Y.; Han, S.; Smith, C.W.; Johnson, R.J.; Wu, J.Y.; et al. Modulation of inflammation by slit protein in vivo in experimental crescentic glomerulonephritis. Am. J. Pathol. 2004, 165, 341–352. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Yuen, D.A.; Bajwa, A.; Huang, Y.W.; Sokollik, C.; Huang, L.; Lam, G.Y.; Tole, S.; Liu, G.Y.; Pan, J.; et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2013, 24, 1274–1287. [Google Scholar] [CrossRef]
- Lobaton, T.; Azuara, D.; Rodriguez-Moranta, F.; Loayza, C.; Sanjuan, X.; de Oca, J.; Fernandez-Robles, A.; Guardiola, J.; Capella, G. Relationship between methylation and colonic inflammation in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 10591–10598. [Google Scholar] [CrossRef]
- Altay, T.; McLaughlin, B.; Wu, J.Y.; Park, T.S.; Gidday, J.M. Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia. Exp. Neurol. 2007, 207, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Sherchan, P.; Huang, L.; Akyol, O.; Reis, C.; Tang, J.; Zhang, J.H. Recombinant Slit2 Reduces Surgical Brain Injury Induced Blood Brain Barrier Disruption via Robo4 Dependent Rac1 Activation in a Rodent Model. Sci. Rep. 2017, 7, 746. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Q.; Sun, X.; Gong, X.; Yang, Y.; Chen, C.; Shan, G. Slit2 ameliorates renal inflammation and fibrosis after hypoxia-and lipopolysaccharide-induced epithelial cells injury in vitro. Exp. Cell Res. 2017, 352, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yuen, D.A.; Huang, Y.W.; Liu, G.Y.; Patel, S.; Fang, F.; Zhou, J.; Thai, K.; Sidiqi, A.; Szeto, S.G.; Chan, L.; et al. Recombinant N-Terminal Slit2 Inhibits TGF-beta-Induced Fibroblast Activation and Renal Fibrosis. J. Am. Soc. Nephrol. 2016, 27, 2609–2615. [Google Scholar] [PubMed]
- Zhao, H.; Anand, A.R.; Ganju, R.K. Slit2-Robo4 pathway modulates lipopolysaccharide-induced endothelial inflammation and its expression is dysregulated during endotoxemia. J. Immunol. 2014, 192, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.Q.; Geng, Z.H.; Ma, L.; Geng, J.G. Slit2 regulates attractive eosinophil and repulsive neutrophil chemotaxis through differential srGAP1 expression during lung inflammation. J. Immunol. 2010, 185, 6294–6305. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Ren, X.R.; Huang, Y.Z.; Xie, Y.; Liu, G.; Saito, H.; Tang, H.; Wen, L.; Brady-Kalnay, S.M.; Mei, L.; et al. Signal transduction in neuronal migration: Roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 2001, 107, 209–221. [Google Scholar] [CrossRef]
- Sherchan, P.; Huang, L.; Wang, Y.; Akyol, O.; Tang, J.; Zhang, J.H. Recombinant Slit2 attenuates neuroinflammation after surgical brain injury by inhibiting peripheral immune cell infiltration via Robo1-srGAP1 pathway in a rat model. Neurobiol. Dis. 2016, 85, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tole, S.; Mukovozov, I.M.; Huang, Y.W.; Magalhaes, M.A.; Yan, M.; Crow, M.R.; Liu, G.Y.; Sun, C.X.; Durocher, Y.; Glogauer, M.; et al. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. J. Leukoc. Biol. 2009, 86, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Slit2 Splicing Variants | n | Diseases | pa | |
|---|---|---|---|---|
| Lung Cancer | Pneumothorax | |||
| No. of subjects | 18 | 54 | ||
| Slit2-WT ≦ Slit2-ΔE15 | 22 | 0 (0.0) | 22 (40.7) | 0.001 |
| Slit2-WT > Slit2-ΔE15 | 50 | 18 (100.0) | 32 (59.3) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-F.; Chuang, C.-Y.; Lin, P.; Chen, W.-T.; Su, S.-E.; Liao, C.-Y.; Jan, M.-S.; Chang, J.T. Lung Tumorigenesis Alters the Expression of Slit2-exon15 Splicing Variants in Tumor Microenvironment. Cancers 2019, 11, 166. https://doi.org/10.3390/cancers11020166
Wu M-F, Chuang C-Y, Lin P, Chen W-T, Su S-E, Liao C-Y, Jan M-S, Chang JT. Lung Tumorigenesis Alters the Expression of Slit2-exon15 Splicing Variants in Tumor Microenvironment. Cancers. 2019; 11(2):166. https://doi.org/10.3390/cancers11020166
Chicago/Turabian StyleWu, Ming-Fang, Cheng-Yen Chuang, Pinpin Lin, Wei-Ting Chen, Shang-Er Su, Chen-Yi Liao, Ming-Shiou Jan, and Jinghua Tsai Chang. 2019. "Lung Tumorigenesis Alters the Expression of Slit2-exon15 Splicing Variants in Tumor Microenvironment" Cancers 11, no. 2: 166. https://doi.org/10.3390/cancers11020166





