Anti-Metastatic Benefits Produced by Hyperthermia and a CCL3 Derivative
Abstract
:1. Introduction
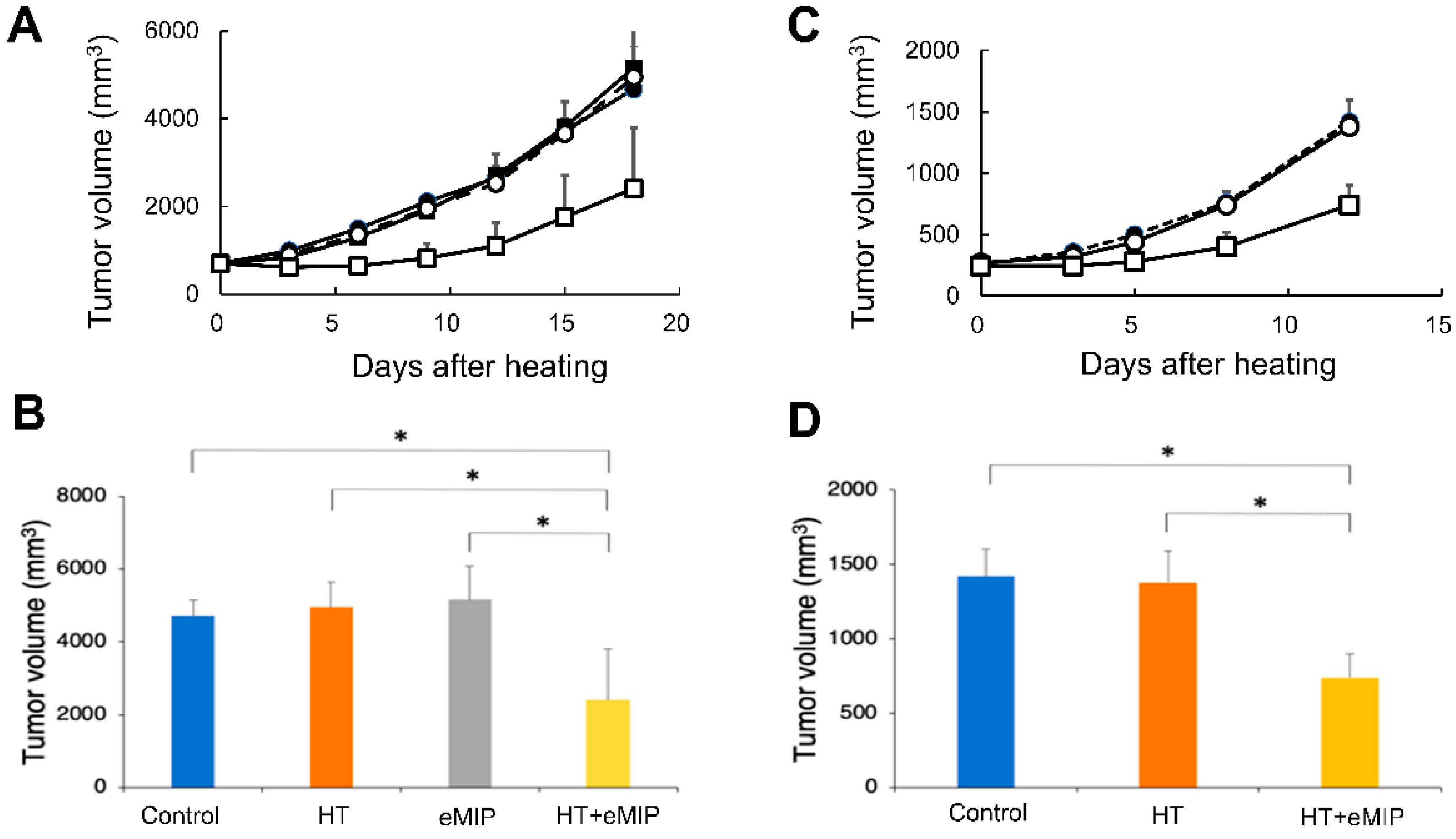
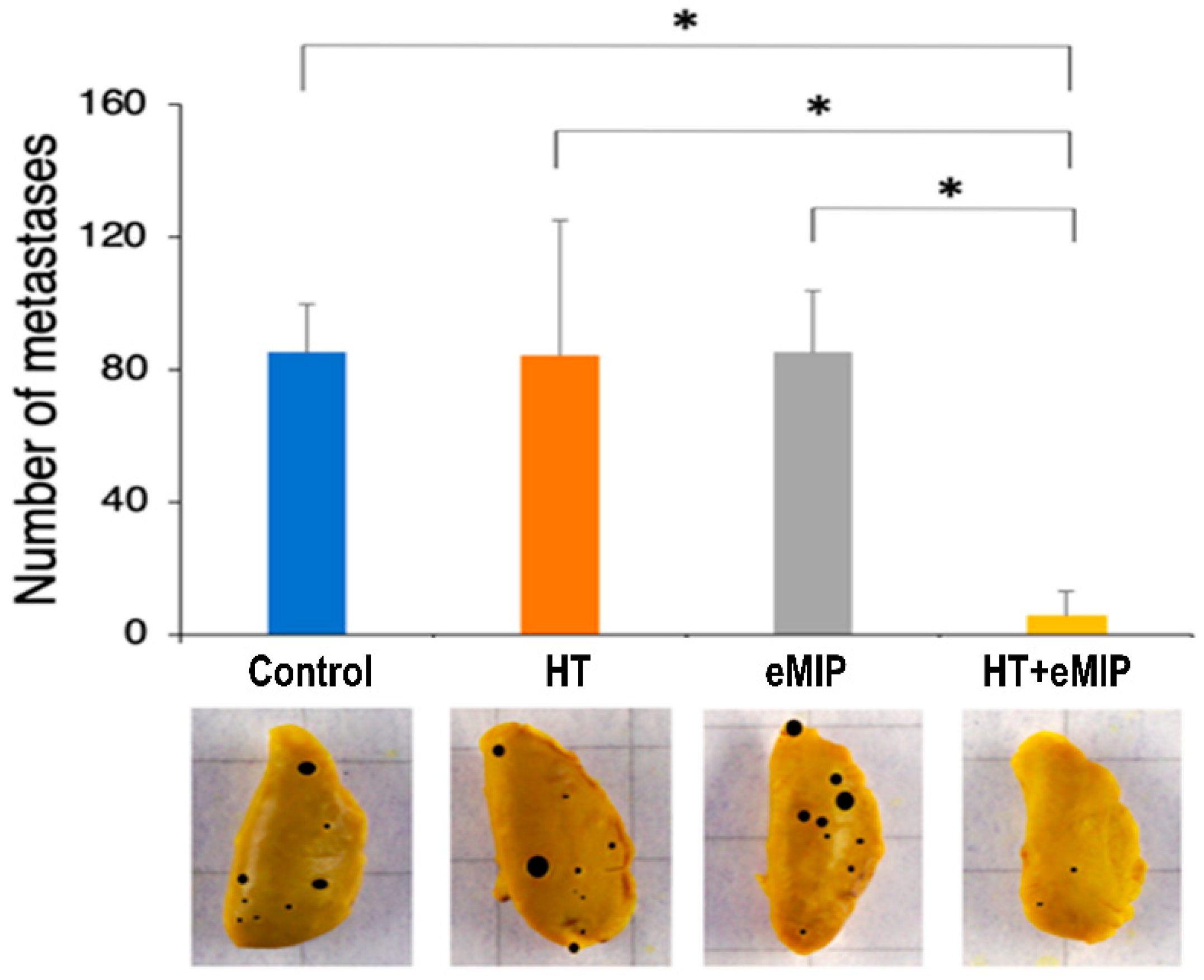
2. Results
2.1. eMIP Administration after Mild Local HT Treatment Induces Anti-Tumor Activity at the Treated Site
2.2. eMIP Administration after HT Treatment Inhibited Lung Metastasis
3. Discussion
4. Materials and Methods
4.1. Preparation of Chemokine
4.2. Mice
4.3. Tumor Cells
4.4. Tumor Growth
4.5. Pulmonary Metastasis Assay
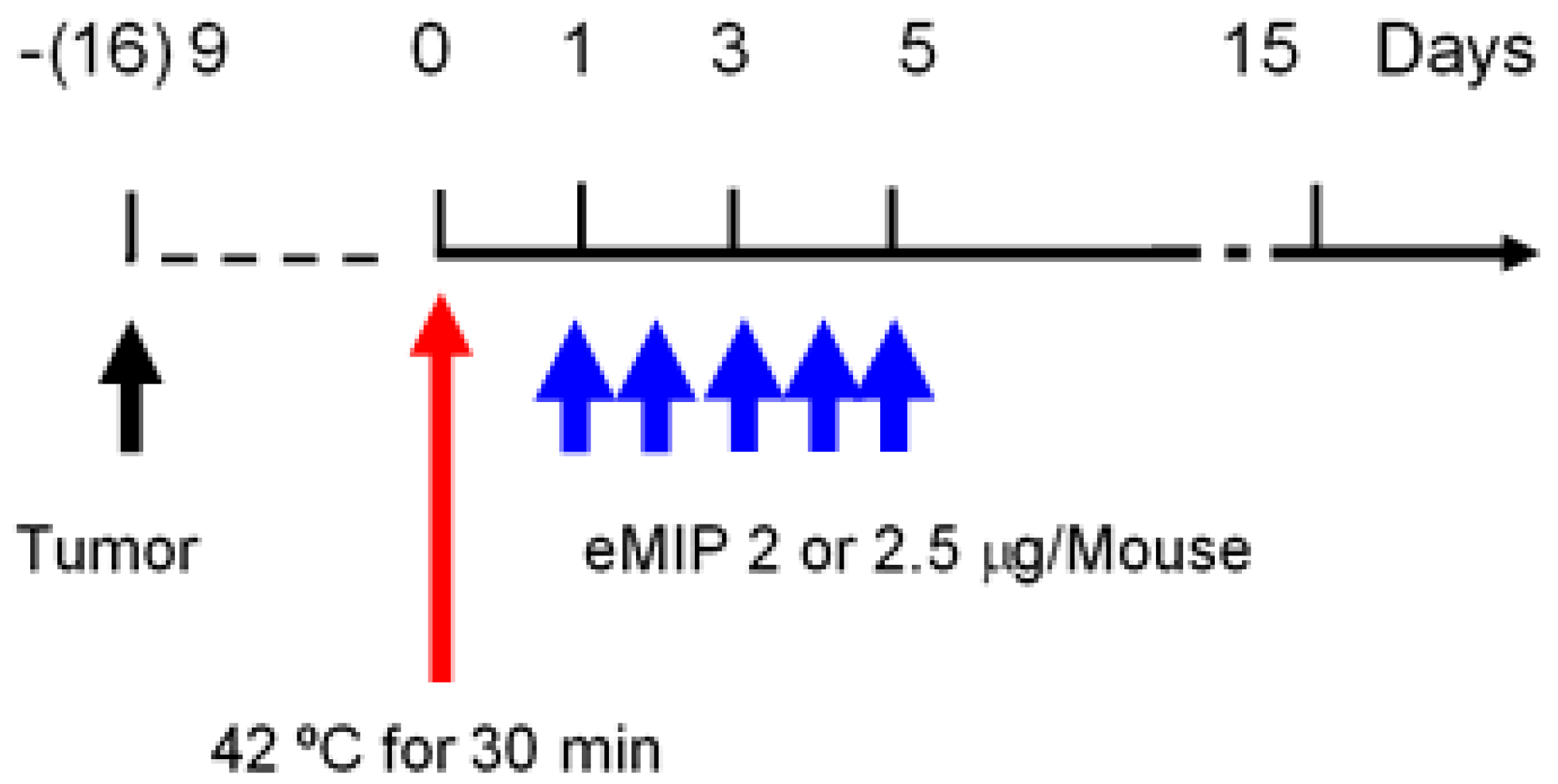
4.6. HT and eMIP Administration
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, S.; van Os, R.; Oei, B.; Poortmans, P. Impact of technique and schedule of reirradiation plus hyperthermia on outcome after surgery for patients with recurrent breast cancer. Cancers (Basel) 2019, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.; Loke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.; Crezee, J. Variation in clinical application of hyperthermic intraperitoneal chemotherapy: A review. Cancers (Basel) 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Hurwitz, M.D.; Krishnan, S.; Asea, A. Combined hyperthermia and radiotherapy for the treatment of cancer. Cancers (Basel) 2011, 3, 3799–3823. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sawaji, Y.; Matsui, N.; Sato, H.; Seiki, M.; Mori, Y.; Ito, A. Heat shock suppresses membrane type 1-matrix metalloproteinase production and progelatinase A activation in human fibrosarcoma HT-1080 cells and thereby inhibits cellular invasion. Biochem. Biophys. Res. Commun. 1999, 265, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Shukla, H.D.; Soman, S.; Samanta, S.; Singh, P.; Kamlapurkar, S.; Saeed, A.; Amin, N.P.; Vujaskovic, Z. Immunotherapy, radiotherapy, and hyperthermia: A combined therapeutic approach in pancreatic cancer treatment. Cancers (Basel) 2018, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Hiniker, S.M.; Maecker, H.T.; Knox, S.J. Predictors of clinical response to immunotherapy with or without radiotherapy. J. Radiat. Oncol. 2015, 4, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Burg, S.H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C.J. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zeng, Y.; Graner, M.W.; Katsanis, E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood 2002, 100, 4108–4115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Weiss, E.M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.W.; Huang, C.C.; Yang, K.L.; Chi, M.S.; Chiang, H.C.; Wang, Y.S.; Andocs, G.; Szasz, A.; Li, W.T.; Chi, K.H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ishiwata, Y.; Nakagawa, K.; Yokochi, S.; Taruki, C.; Akuta, T.; Ohtomo, K.; Matsushima, K.; Tamatani, T.; Kanegasaki, S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin. Cancer Res. 2008, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Kanegasaki, S.; Matsushima, K.; Shiraishi, K.; Nakagawa, K.; Tsuchiya, T. Macrophage inflammatory protein derivative ECI301 enhances the alarmin-associated abscopal benefits of tumor radiotherapy. Cancer Res. 2014, 74, 5070–5078. [Google Scholar] [CrossRef] [PubMed]
- Adkins, I.; Sadilkova, L.; Hradilova, N.; Tomala, J.; Kovar, M.; Spisek, R. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology 2017, 6, e1311433. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B. The war on cancer. Lancet 1996, 347, 1377–1381. [Google Scholar] [CrossRef]
- Shoji, H.; Motegi, M.; Takakusagi, Y.; Asao, T.; Kuwano, H.; Takahashi, T.; Ogoshi, K. Chemoradiotherapy and concurrent radiofrequency thermal therapy to treat primary rectal cancer and prediction of treatment responses. Oncol. Rep. 2017, 37, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Application of heat to cancer chemotherapy--experimental studies. Nagoya J. Med. Sci. 1967, 30, 1–21. [Google Scholar] [PubMed]
- Dickson, J.A.; Suzangar, M. In vitro-in vivo studies on the susceptibility of the solid Yoshida sarcoma to drugs and hyperthermia (42 degrees). Cancer Res. 1974, 34, 1263–1274. [Google Scholar] [PubMed]
- Barlogie, B.; Corry, P.M.; Drewinko, B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res. 1980, 40, 1165–1168. [Google Scholar] [PubMed]
- Shiu, M.H.; Cahan, A.; Fogh, J.; Fortner, J.G. Sensitivity of xenografts of human pancreatic adenocarcinoma in nude mice to heat and heat combined with chemotherapy. Cancer Res. 1983, 43, 4014–4018. [Google Scholar] [PubMed]
- Tsumura, M.; Yoshiga, K.; Takada, K. Enhancement of antitumor effects of 1-hexylcarbamoyl-5-fluorouracil combined with hyperthermia on Ehrlich ascites tumor in vivo and Nakahara-Fukuoka sarcoma cell in vitro. Cancer Res. 1988, 48, 3977–3980. [Google Scholar] [PubMed]
- Hahn, G.M. Responses of murine tumors and normal tissues. In Hyperthermia and Cancer; Hahn, G.M., Ed.; Springer: Boston, MA, USA, 1982; pp. 131–177. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Kambe, R.; Tsuchiya, T.; Kanegasaki, S.; Takahashi, A. Anti-Metastatic Benefits Produced by Hyperthermia and a CCL3 Derivative. Cancers 2019, 11, 1770. https://doi.org/10.3390/cancers11111770
Ma L, Kambe R, Tsuchiya T, Kanegasaki S, Takahashi A. Anti-Metastatic Benefits Produced by Hyperthermia and a CCL3 Derivative. Cancers. 2019; 11(11):1770. https://doi.org/10.3390/cancers11111770
Chicago/Turabian StyleMa, Liqiu, Ryosuke Kambe, Tomoko Tsuchiya, Shiro Kanegasaki, and Akihisa Takahashi. 2019. "Anti-Metastatic Benefits Produced by Hyperthermia and a CCL3 Derivative" Cancers 11, no. 11: 1770. https://doi.org/10.3390/cancers11111770






