Hybrid 18F-FDG-PET/MRI Measurement of Standardized Uptake Value Coupled with Yin Yang 1 Signature in Metastatic Breast Cancer. A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Sample Collection
2.3. RNA Extraction and Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assay
2.4. YY1 Enzyme-Linked Immunosorbent Assay (ELISA) Test
2.5. Hybrid 18F-FDG-PET/MRI Technique
2.6. Image Analysis
2.7. Statistical Analysis
3. Results
3.1. Study Population and Imaging Features
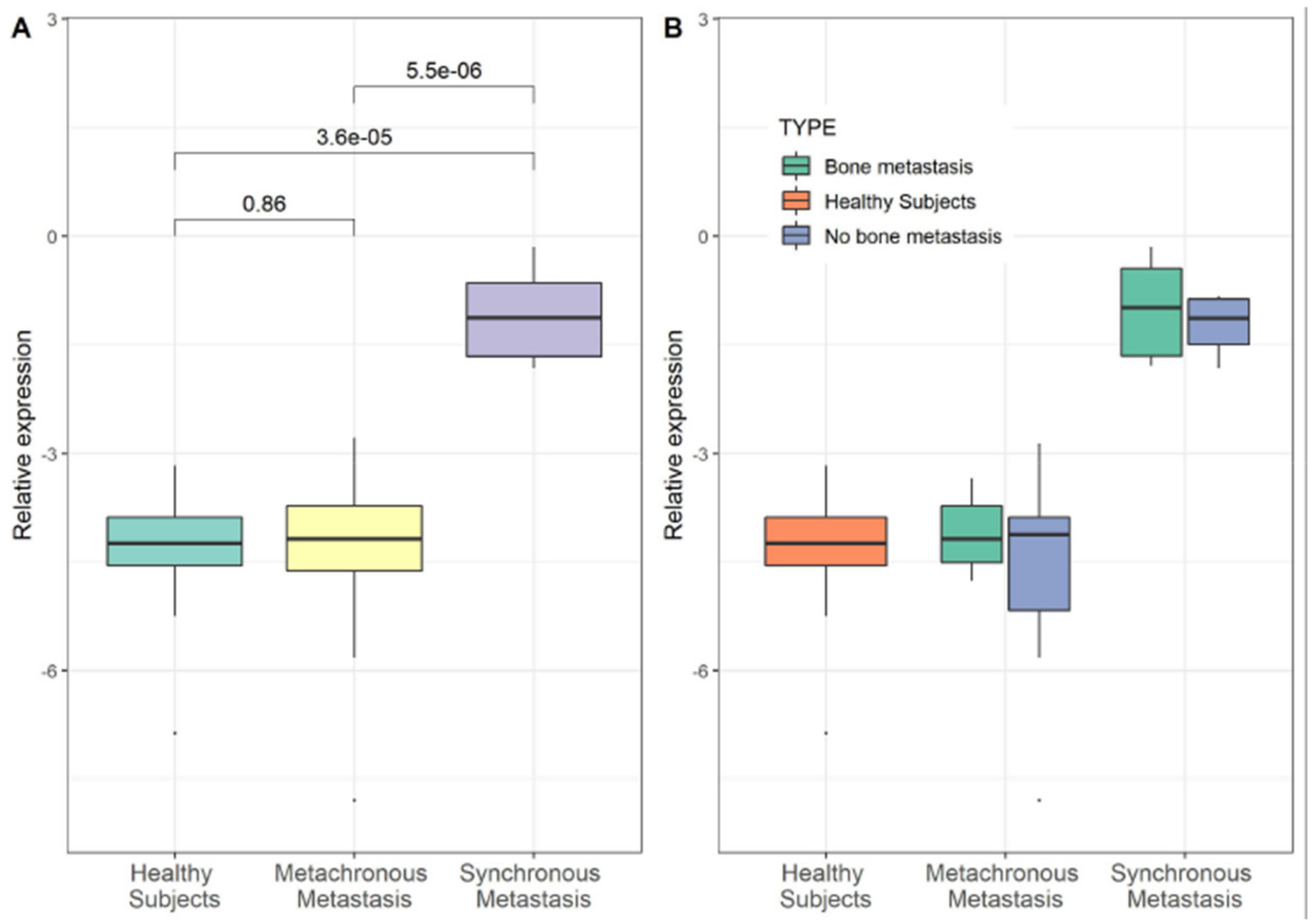
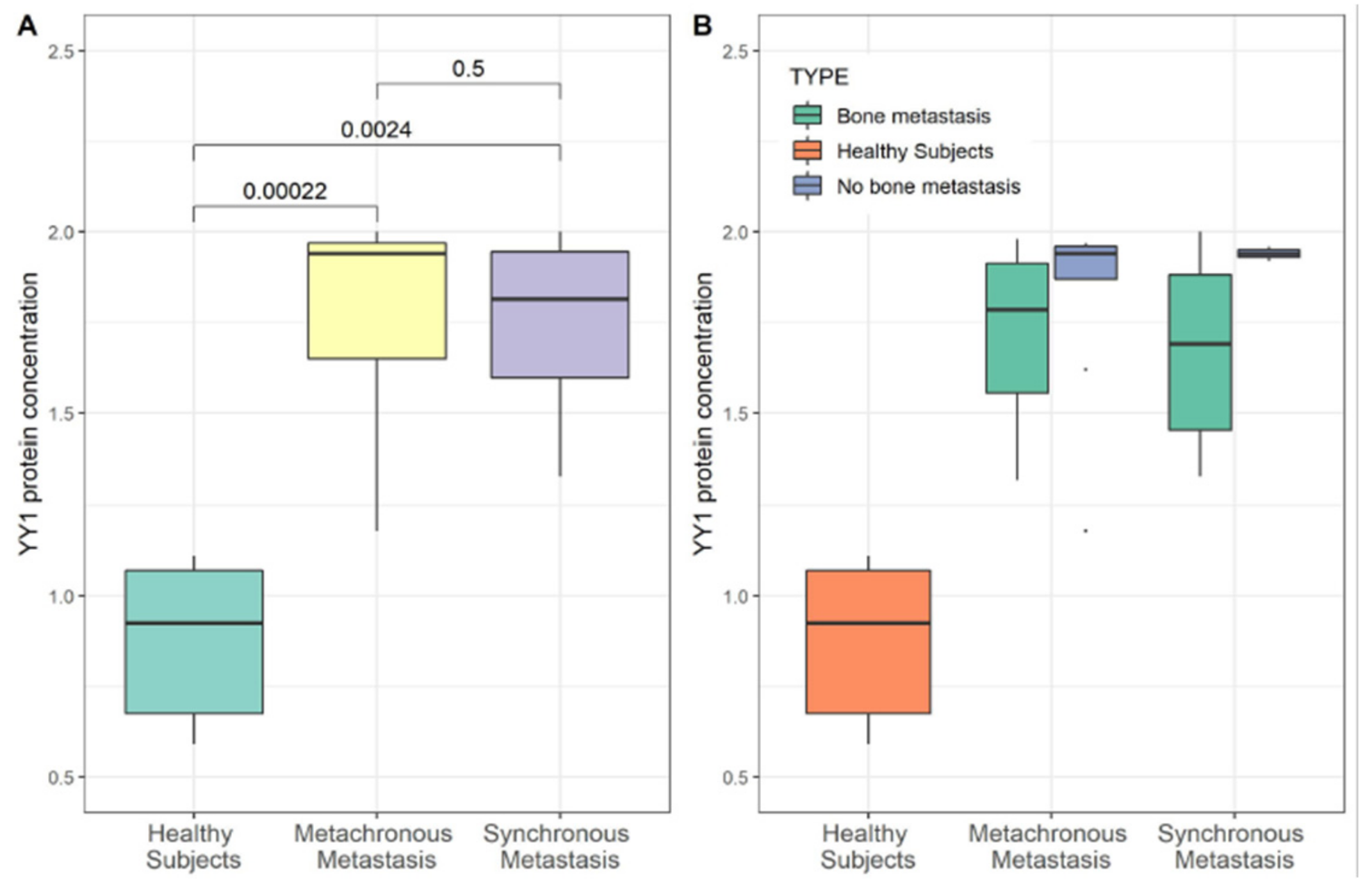
3.2. YY1 Molecular Expression in Breast Cancer (BC) Patient
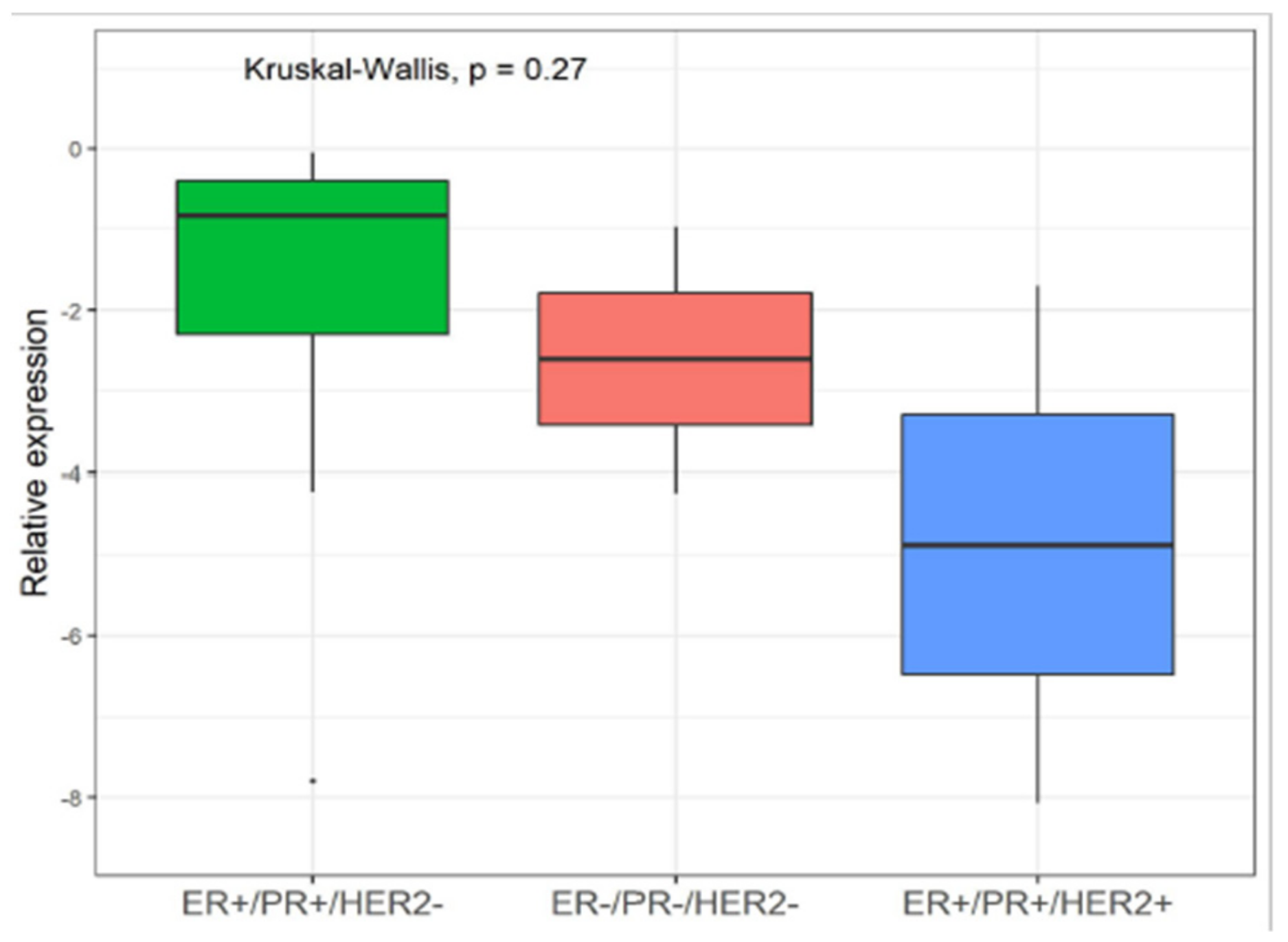
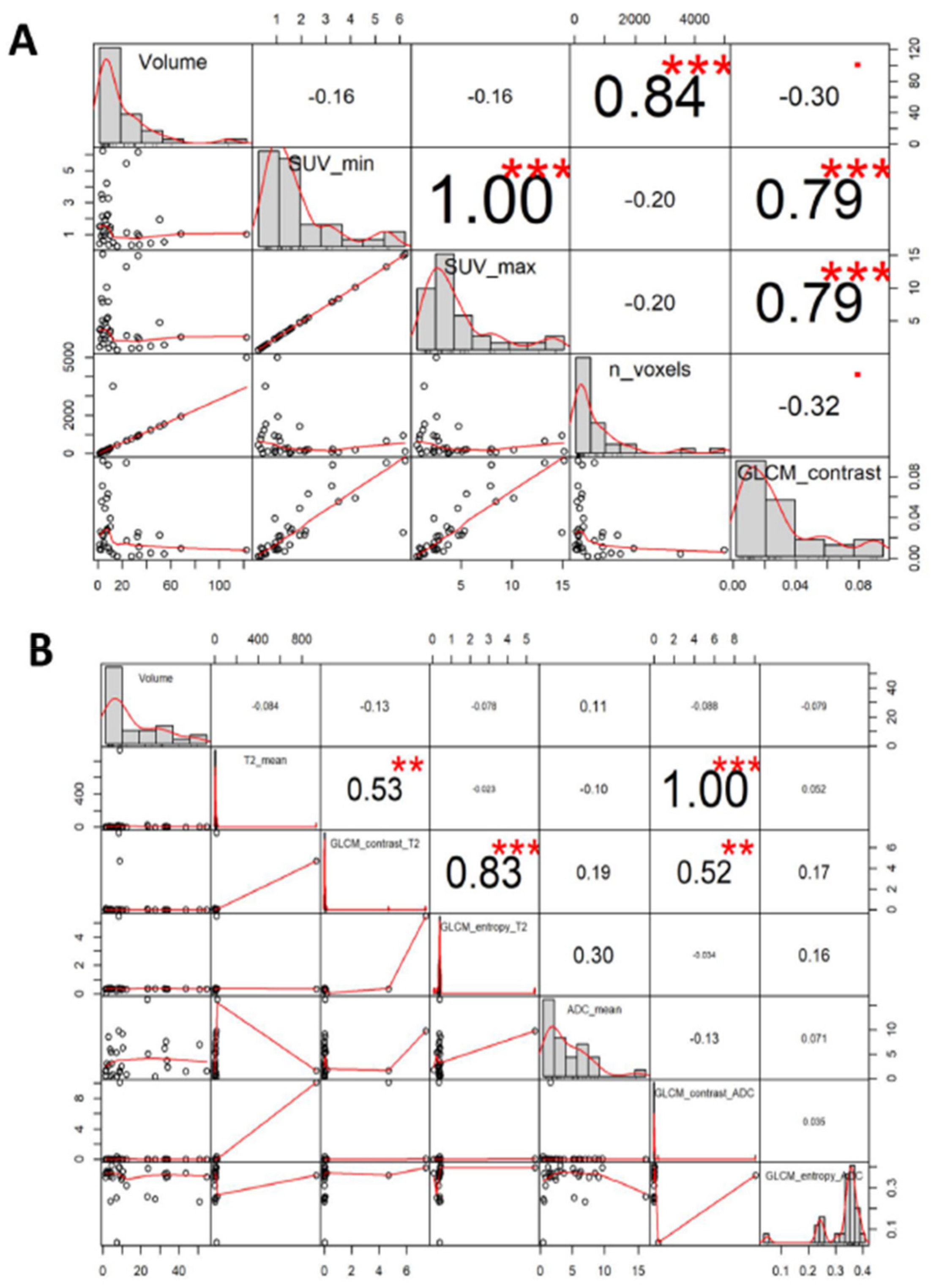
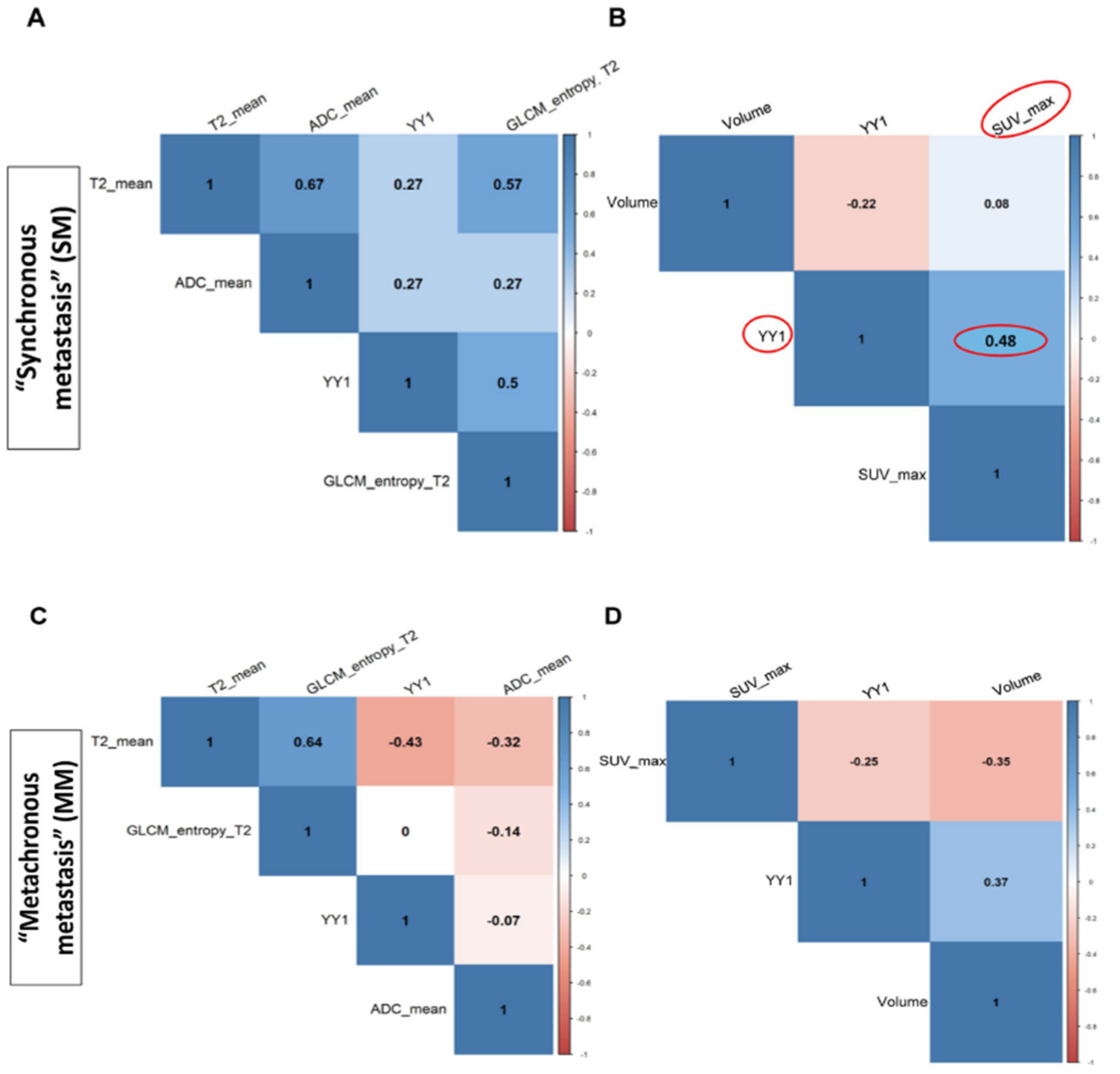
3.3. Correlation Between YY1 and FDG-PET/MRI in BC Patients
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| BC | Breast cancer |
| DWI | Diffusion Weighted Imaging |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ER | Estrogen Receptor |
| 18F-FDG-PET/MRI | Fluorine-18-fluorodeoxyglucose Positron Emission Tomography/ Magnetic Risonance Imaging |
| GLCM | Gray Level Co-occurrence Matrix |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HS | Healthy subjects |
| IHC | Immunohistochemistry |
| MM | Metachronous Metastasis |
| PBMCs | Peripheral blood mononuclear cells |
| PET/CT | Positron Emission Tomography/ Computed Tomography |
| PR | Progesteron Receptor |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SM | Synchronous Metastasis |
| SUV | Standardized Uptake Value |
| VOI | Volume of interest |
| YY1 | YY1 Transcription Factor |
References
- Schiano, C.; Soricelli, A.; De Nigris, F.; Napoli, C. New challenges in integrated diagnosis by imaging and osteo-immunology in bone lesions. Expert Rev. Clin. Immunol. 2018, 15, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.K.; Schmidt, H.; Schwenzer, N.F. MR/PET: A New Challenge in Hybrid Imaging. Am. J. Roentgenol. 2012, 199, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, H.P.; Pichler, B.J.; Krieg, R.; Heiss, W.D. An integrated MR/PET system: prospective applications. Abdom. Imaging 2009, 34, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, D.; Leal, M.P.; Garcia-Bocanegra, I.; Garcia-Martin, M.L.; García-Bocanegra, I.; García-Martín, M.L. Molecular imaging of breast cancer: present and future directions. Front. Chem. 2014, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.; Whitehouse, H.; Naidoo, K.; Byers, R. Yin Yang 1 in human cancer. Crit. Rev. Oncog. 2011, 16, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Spandidos, D.A. Yin Yang 1 expression in human tumors. Cell Cycle 2010, 9, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Stovall, D.B.; Inoue, K.; Sui, G. The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 2011, 16, 163–197. [Google Scholar] [CrossRef]
- Castellano, G.; Torrisi, E.; Ligresti, G.; Malaponte, G.; McCubrey, J.A.; Nicoletti, F.; Travali, S.; Canevari, S.; Libra, M. Yin Yang 1 overexpression in diffuse large B-cell lymphoma is associated with B-cell transformation and tumor progression. Cell Cycle 2010, 9, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Baritaki, S.; Huerta-Yepez, S.; Sakai, T.; Spandidos, D.A.; Bonavida, B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol. Cancer Ther. 2007, 6, 1387–1399. [Google Scholar] [CrossRef]
- De Nigris, F.; Zanella, L.; Cacciatore, F.; De Chiara, A.; Fazioli, F.; Chiappetta, G.; Apice, G.; Infante, T.; Monaco, M.; Rossiello, R.; et al. YY1 overexpression is associated with poor prognosis and metastasis-free survival in patients suffering osteosarcoma. BMC Cancer 2011, 11, 472. [Google Scholar] [CrossRef]
- Seligson, D.; Horvath, S.; Huerta-Yepez, S.; Hanna, S.; Garban, H.; Roberts, A.; Shi, T.; Liu, X.; Chia, D.; Goodglick, L.; et al. Expression of transcription factor Yin Yang 1 in prostate cancer. Int. J. Oncol. 2005, 27, 131–141. [Google Scholar] [CrossRef]
- De Nigris, F.; Rossiello, R.; Schiano, C.; Arra, C.; Williams-Ignarro, S.; Barbieri, A.; Lanza, A.; Balestrieri, A.; Giuliano, M.T.; Ignarro, L.J.; et al. Deletion of Yin Yang 1 protein in osteosarcoma cells on cell invasion and CXCR4/angiogenesis and metastasis. Cancer Res. 2008, 68, 1797–1808. [Google Scholar] [CrossRef]
- De Nigris, F.; Botti, C.; de Chiara, A.; Rossiello, R.; Apice, G.; Fazioli, F.; Fiorito, C.; Sica, V.; Napoli, C. Expression of transcription factor Yin Yang 1 in human osteosarcomas. Eur. J. Cancer 2006, 42, 2420–2424. [Google Scholar] [CrossRef]
- Chinnappan, D.; Ratnasari, A.; Andry, C.; King, T.C.; Xiao, D.; Weber, H.C. Transcription factor YY1 expression in human gastrointestinal cancer cells. Int. J. Oncol. 2009, 34, 1417–1423. [Google Scholar] [PubMed]
- Allouche, A.; Nolens, G.; Tancredi, A.; Delacroix, L.; Mardaga, J.; Fridman, V.; Winkler, R.; Boniver, J.; Delvenne, P.; Begon, D.Y. The combined immunodetection of AP-2alpha and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Res. 2008, 10, R9. [Google Scholar] [CrossRef]
- Begon, D.Y.; Delacroix, L.; Vernimmen, D.; Jackers, P.; Winkler, R. Yin Yang 1 Cooperates with Activator Protein 2 to StimulateERBB2Gene Expression in Mammary Cancer Cells. J. Biol. Chem. 2005, 280, 24428–24434. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, A.S. YY1 as a regulator of replication-dependent hamster histone H3.2 promoter and an interactive partner of AP-2. J. Biol. Chem. 2001, 276, 28–34. [Google Scholar] [CrossRef]
- Thomassen, M.; Tan, Q.; Kruse, T.A. Gene expression meta-analysis identifies metastatic pathways and transcription factors in breast cancer. BMC Cancer 2008, 8, 394. [Google Scholar] [CrossRef]
- Lieberthal, J.G.; Kaminsky, M.; Parkhurst, C.N.; Tanese, N. The role of YY1 in reduced HP1alpha gene expression in invasive human breast cancer cells. Breast Cancer Res. 2009, 11, 42. [Google Scholar] [CrossRef]
- Shen, X.; Zhong, J.; Yu, P.; Zhao, Q.; Huang, T. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem. Biophys. Res. Commun. 2019, 509, 448–454. [Google Scholar] [CrossRef]
- Hoefnagel, L.D.; Moelans, C.B.; Meijer, S.L.; van Slooten, H.J.; Wesseling, P.; Wesseling, J.; Westenend, P.J.; Bart, J.; Seldenrijk, C.A.; Nagtegaal, I.D.; et al. Prognostic value of estrogen receptor alpha and progesterone receptor conversion in distant breast cancer metastases. Cancer 2012, 118, 4929–4935. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. Breast Cancer Staging, 7th ed.; American Joint Committee on Cancer: Chicago, IL, USA, 2009. [Google Scholar]
- Rienzo, M.; Schiano, C.; Casamassimi, A.; Grimaldi, V.; Infante, T.; Napoli, C. Identification of valid reference housekeeping genes for gene expression analysis in tumor neovascularization studies. Clin. Transl. Oncol. 2013, 15, 211–218. [Google Scholar] [CrossRef]
- Mirabelli, P.; Incoronato, M.; Coppola, L.; Infante, T.; Parente, C.A.; Nicolai, E.; Soricelli, A.; Salvatore, M. SDN Biobank: Bioresource of Human Samples Associated with Functional and/or Morphological Bioimaging Results for the Study of Oncological, Cardiological, Neurological, and Metabolic Diseases. Open J. Bioresour. 2017, 4, 356. [Google Scholar] [CrossRef]
- Daye, D.; Signore, A.; Iannace, C.; Vangel, M.; Luongo, A.; Filomena, M.; Mansi, L.; Salvatore, M.; Fuin, N.; Catana, C.; et al. Staging performance of whole-body DWI, PET/CT and PET/MRI in invasive ductal carcinoma of the breast. Int. J. Oncol. 2017, 51, 281–288. [Google Scholar]
- Pace, L.; Nicolai, E.; Luongo, A.; Aiello, M.; Catalano, O.A.; Soricelli, A.; Salvatore, M. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: Lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur. J. Radiol. 2014, 83, 289–296. [Google Scholar] [CrossRef]
- Catalano, O.A.; Nicolai, E.; Rosen, B.R.; Luongo, A.; Catalano, M.; Iannace, C.; Guimarães, A.; Vangel, M.G.; Mahmood, U.; Soricelli, A.; et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br. J. Cancer 2015, 112, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Ehinger, A.; Malmström, P.; Bendahl, P.O.; Elston, C.W.; Falck, A.K.; Forsare, C.; Grabau, D.; Rydén, L.; Stål, O.; Fernö, M.; et al. Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013. Acta Oncol. 2017, 56, 68–74. [Google Scholar] [CrossRef]
- Lee, M.H.; Lahusen, T.; Wang, R.H.; Xiao, C.; Xu, X.; Hwang, Y.S.; He, W.W.; Shi, Y.; Deng, C.X. Yin Yang 1 positively regulates BRCA1 and inhibits mammary cancer formation. Oncogene 2012, 31, 116–127. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Wang, M.Y.; Huang, H.Y.; Kuo, Y.L.; Lo, C.; Sun, H.Y.; Lyu, Y.J.; Chen, B.R.; Li, J.N.; Chen, P.S. TARBP2-Enhanced Resistance during Tamoxifen Treatment in Breast Cancer. Cancers 2019, 11, 210. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.G.; Cronin, K.A. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.; Huang, W.; Kute, T.E.; Miller, L.D.; Zhang, Q.; Hatcher, H.; Wang, J.; Stovall, D.B.; Russell, G.B.; Cao, P.D.; et al. Yin Yang 1 plays an essential role in breast cancer and negatively regulates p27. Am. J. Pathol. 2012, 180, 2120–2133. [Google Scholar] [CrossRef]
- Wang, A.M.; Huang, T.T.; Hsu, K.W.; Huang, K.H.; Fang, W.L.; Yang, M.H.; Lo, S.S.; Chi, C.W.; Lin, J.J.; Yeh, T.S. Yin Yang 1 is a target of microRNA-34 family and contributes to gastric carcinogenesis. Oncotarget 2014, 5, 5002–5016. [Google Scholar] [CrossRef]
- Kang, W.; Tong, J.H.; Chan, A.W.; Zhao, J.; Dong, Y.; Wang, S.; Yang, W.; Mc Sin, F.; Ng, S.S.; Yu, J.; et al. Yin Yang 1 contributes to gastric carcinogenesis and its nuclear expression correlates with shorter survival in patients with early stage gastric adenocarcinoma. J. Transl. Med. 2014, 12, 80. [Google Scholar] [CrossRef]
- Bonavida, B.; Kaufhold, S. Prognostic significance of YY1 protein expression and mRNA levels by bioinformatics analysis in human cancers: A therapeutic target. Pharmacol. Ther. 2015, 150, 149–168. [Google Scholar] [CrossRef]
- Shi, J.; Hao, A.; Zhang, Q.; Sui, G. The role of YY1 in oncogenesis and its potential as a drug target in cancer therapies. Curr. Cancer Drug Targets 2015, 15, 145–157. [Google Scholar] [CrossRef]
- Yang, W.; Feng, B.; Meng, Y.; Wang, J.; Geng, B.; Cui, Q.; Zhang, H.; Yang, Y.; Yang, J. FAM3C-YY1 axis is essential for TGFβ-promoted proliferation and migration of human breast cancer MDA-MB-231 cells via the activation of HSF1. J. Cell. Mol. Med. 2019, 23, 3464–3475. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Wang, Z.; Zhang, T.; Wu, P.; Huang, J. Yin-yang effect of tumor infiltrating B cells in breast cancer: From mechanism to immunotherapy. Cancer Lett. 2017, 393, 1–7. [Google Scholar] [CrossRef]
- Powe, D.G.; Akhtar, G.; Habashy, H.O.; Abdel-Fatah, T.M.; Rakha, E.A.; Green, A.R.; O Ellis, I. Investigating AP-2 and YY1 protein expression as a cause of high HER2 gene transcription in breast cancers with discordant HER2 gene amplification. Breast Cancer Res. 2009, 11, R90. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Huang, E.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Zuley, M.; Net, J.M.; et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016, 2, 16012. [Google Scholar] [CrossRef]
- Grueneisen, J.; Sawicki, L.M.; Wetter, A.; Kirchner, J.; Kinner, S.; Aktas, B.; Forsting, M.; Ruhlmann, V.; Umutlu, L. Evaluation of PET and MR datasets in integrated 18F-FDG PET/MRI: A comparison of different MR sequences for whole-body restaging of breast cancer patients. Eur. J. Radiol. 2017, 89, 14–19. [Google Scholar] [CrossRef]
- Rahim, M.K.; Kim, S.E.; So, H.; Kim, H.J.; Cheon, G.J.; Lee, E.S.; Kang, K.W.; Lee, D.S. Recent Trends in PET Image Interpretations Using Volumetric and Texture-based Quantification Methods in Nuclear Oncology. Nucl. Med. Mol. Imaging 2014, 48, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Cho, S.F.; Chen, Y.W.; Tu, H.P.; Lin, C.Y.; Chang, C.S. SUV on Dual-Phase FDG PET/CT Correlates With the Ki-67 Proliferation Index in Patients With Newly Diagnosed Non-Hodgkin Lymphoma. Clin. Nucl. Med. 2012, 37, e189–e195. [Google Scholar] [CrossRef]
- Güth, U.; Magaton, I.; Huang, D.J.; Fisher, R.; Schötzau, A.; Vetter, M. Primary and secondary distant metastatic breast cancer: Two sides of the same coin. Breast 2014, 23, 26–32. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 2015, 32, 125–133. [Google Scholar] [CrossRef]
- Kast, K.; Link, T.; Friedrich, K.; Petzold, A.; Niedostatek, A.; Schoffer, O.; Werner, C.; Klug, S.J.; Werner, A.; Gatzweiler, A.; et al. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res. Treat. 2015, 150, 621–629. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Bourgeois, M.; Gouard, S.; Ansquer, C.; Barbaud, M.; Sébille, J.C.; Chérel, M.; Kraeber-Bodéré, F.; Carlier, T. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers 2019, 11, 1282. [Google Scholar] [CrossRef]
- Jain, A.; Dubey, I.; Chauhan, M.; Kumar, R.; Agarwal, S.; Kishore, B.; Vishnoi, M.; Paliwal, D.; John, A.; Kumar, N.; et al. Tumor characteristics and metabolic quantification in carcinoma breast: An institutional experience. Indian J. Cancer 2017, 54, 333. [Google Scholar] [CrossRef]


| Number or Values | |
|---|---|
| Total Cohort | 52 |
| Healthy Subjects “Control group” | 15 |
| Breast cancer group “Synchronous metastasis” (SM) | 11 |
| Breast cancer group “Metachronous metastasis” (MM) | 26 |
| Mean age (years) | 57 |
| Range (min–max) | 32–88 |
| TNM | 13 |
| T0 | 1 |
| T1 | 2 |
| T2 | 7 |
| T3 | 3 |
| NA | 24 |
| Tumor Grade | 10 |
| G2 | 8 |
| G3 | 2 |
| NA | 27 |
| Ki 67 Status a | 12 |
| Low | 4 |
| High | 8 |
| NA | 25 |
| ER Status | 15 |
| Negative | 3 |
| Positive | 12 |
| NA | 22 |
| PR Status | 15 |
| Negative | 3 |
| Positive | 12 |
| NA | 22 |
| Her2 Status b | 13 |
| Negative | 9 |
| Positive | 3 |
| NA | 24 |
| Synchronous Metastasis Group | |||||
| Features | Mean ± SD | Median | Min | Max | Range |
| Volume | 9.54 ± 7.06 | 8.73 | 1.91 | 23.30 | 21.39 |
| SUV_max | 5.16 ± 4.19 | 3.78 | 1.33 | 13.24 | 11.91 |
| n_voxels | 721.86 ± 1247.68 | 247.00 | 54.00 | 3516.00 | 3462.00 |
| Statistic_Energy | 1.01 ± 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| GLCM_contrast | 0.04 ± 0.03 | 0.03 | 0.00 | 0.09 | 0.09 |
| Metachronous Metastasis Group | |||||
| Features | Mean ± SD | Median | Min | Max | Range |
| Volume | 27.76 ± 40.35 | 7.21 | 2.86 | 122.19 | 119.33 |
| SUV_max | 5.26 ± 4.88 | 2.54 | 1.19 | 15.14 | 13.95 |
| n_voxels | 945.78 ± 1617.10 | 142.00 | 81.00 | 4998.00 | 4917.00 |
| Statistic_Energy | 1.01 ± 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| GLCM_contrast | 0.03 ± 0.03 | 0.02 | 0.00 | 0.10 | 0.09 |
| Synchronous Metastasis Group | |||||
| Features | Mean ± SD | Median | Min | Max | Range |
| Volume | 9.33 ± 6.40 | 8.73 | 1.56 | 23.30 | 21.75 |
| T2_mean | 8.72 ± 8.22 | 6.58 | 0.16 | 24.81 | 24.65 |
| GLCM_contrast_T2 | 1.87 ± 2.45 | 0.03 | 0.00 | 7.39 | 7.39 |
| GLCM_entropy_T2 | 1.87 ± 1.72 | 0.39 | 0.06 | 5.47 | 5.41 |
| B_value | 5.75 ± 4.71 | 4.42 | 0.02 | 14.54 | 14.52 |
| ADC_mean | 5.94 ± 5.06 | 5.29 | 0.82 | 16.23 | 15.40 |
| GLCM_contrast_ADC | 1.01 ± 0.02 | 0.02 | 0.00 | 0.07 | 0.06 |
| GLCM_entropy_ADC | 1.34 ± 0.06 | 0.37 | 0.25 | 0.40 | 0.15 |
| Metachronous Metastasis Group | |||||
| Features | Mean ± SD | Median | Min | Max | Range |
| Volume | 17.15 ± 22.04 | 5.02 | 2.86 | 54.53 | 51.67 |
| T2_mean | 5.16 ± 5.71 | 2.09 | 1.18 | 15.74 | 14.56 |
| GLCM_contrast_T2 | 1.01 ± 0.03 | 0.01 | 0.00 | 0.09 | 0.08 |
| GLCM_entropy_T2 | 1.35 ± 0.03 | 0.36 | 0.31 | 0.41 | 0.10 |
| B_value | 4.07 ± 4.14 | 1.88 | 1.44 | 11.58 | 10.14 |
| ADC_mean | 2.97 ± 2.94 | 1.53 | 0.45 | 8.50 | 8.04 |
| GLCM_contrast_ADC | 1.07 ± 0.15 | 0.01 | 0.00 | 0.41 | 0.40 |
| GLCM_entropy_ADC | 0.32 ± 0.13 | 0.37 | 0.04 | 0.38 | 0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiano, C.; Franzese, M.; Pane, K.; Garbino, N.; Soricelli, A.; Salvatore, M.; de Nigris, F.; Napoli, C. Hybrid 18F-FDG-PET/MRI Measurement of Standardized Uptake Value Coupled with Yin Yang 1 Signature in Metastatic Breast Cancer. A Preliminary Study. Cancers 2019, 11, 1444. https://doi.org/10.3390/cancers11101444
Schiano C, Franzese M, Pane K, Garbino N, Soricelli A, Salvatore M, de Nigris F, Napoli C. Hybrid 18F-FDG-PET/MRI Measurement of Standardized Uptake Value Coupled with Yin Yang 1 Signature in Metastatic Breast Cancer. A Preliminary Study. Cancers. 2019; 11(10):1444. https://doi.org/10.3390/cancers11101444
Chicago/Turabian StyleSchiano, Concetta, Monica Franzese, Katia Pane, Nunzia Garbino, Andrea Soricelli, Marco Salvatore, Filomena de Nigris, and Claudio Napoli. 2019. "Hybrid 18F-FDG-PET/MRI Measurement of Standardized Uptake Value Coupled with Yin Yang 1 Signature in Metastatic Breast Cancer. A Preliminary Study" Cancers 11, no. 10: 1444. https://doi.org/10.3390/cancers11101444
APA StyleSchiano, C., Franzese, M., Pane, K., Garbino, N., Soricelli, A., Salvatore, M., de Nigris, F., & Napoli, C. (2019). Hybrid 18F-FDG-PET/MRI Measurement of Standardized Uptake Value Coupled with Yin Yang 1 Signature in Metastatic Breast Cancer. A Preliminary Study. Cancers, 11(10), 1444. https://doi.org/10.3390/cancers11101444





