The Emerging Roles of Cancer Stem Cells and Wnt/Beta-Catenin Signaling in Hepatoblastoma
Abstract
:1. Introduction
2. Molecular Signatures Associated with HB
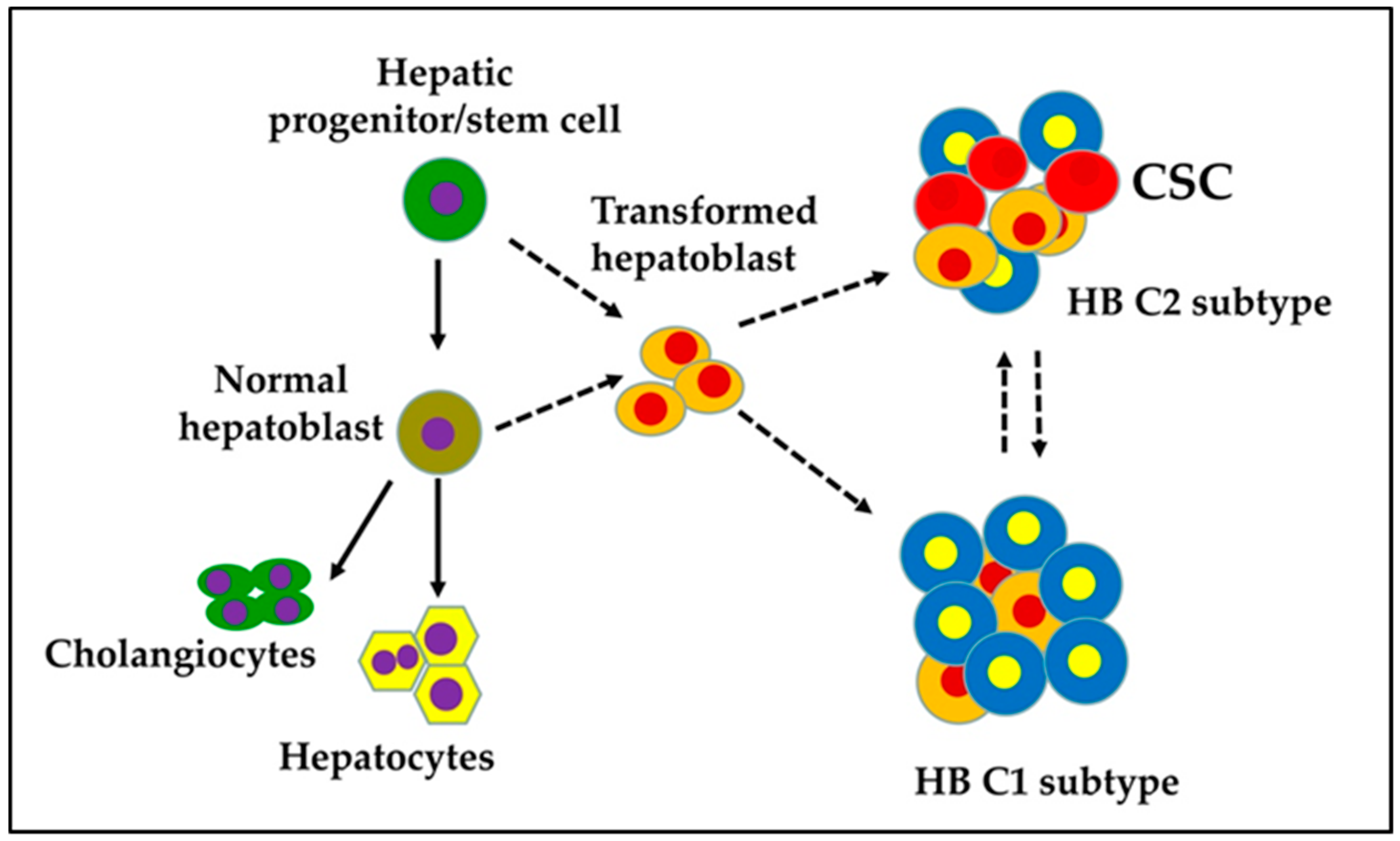
3. Cellular Origin of HB: Transformed Hepatoblasts
4. Epithelial–Mesenchymal Transition in HB
5. Wnt/Beta-Catenin Signaling in HB
6. MicroRNA Regulation of Beta-Catenin Signaling in HB
7. Therapeutic Targeting of Cancer Stem Cells
8. Conclusions
Funding
Conflicts of Interest
References
- Czauderna, P.; Lopez-Terrada, D.; Hiyama, E.; Häberle, B.; Malogolowkin, M.H.; Meyers, R.L. Hepatoblastoma state of the art: Pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Ranganathan, S.; Tao, J.; Monga, S.P. Novel advances in understanding of molecular pathogenesis of hepatoblastoma: A Wnt/Beta-catenin perspective. Gene Expr. 2017, 17, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Linabery, A.M.; Ross, J.A. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 2008, 112, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, M.; Matsui, I.; Abe, J.; Ikeda, H.; Kobayashi, N.; Ohira, M.; Kaneko, M.; Yokoyama, M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998, 58, 3032–3035. [Google Scholar] [PubMed]
- Ikeda, H.; Matsuyama, S.; Tanimura, M. Association between hepatoblastoma and very low birth weight: A trend or a chance? J. Pediatr. 1997, 130, 557–560. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006, 163, 818–828. [Google Scholar] [CrossRef]
- Taat, F.; Bosman, D.K.; Aronson, D.C. Hepatoblastoma in a girl with biliary atresia: Coincidence or co-incidence. Pediatr. Blood Cancer 2004, 43, 603–605. [Google Scholar] [CrossRef]
- Davies, J.Q.; De La Hall, P.M.; Kaschula, R.O.C.; Sinclair-Smith, C.C.; Hartley, P.; Rode, H.; Millar, A.J.W. Hepatoblastoma—Evolution of management and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases. J. Pediatr. Surg. 2004, 39, 1321–1327. [Google Scholar] [CrossRef]
- Haas, J.E.; Muczynski, K.A.; Krailo, M.; Ablin, A.; Land, V.; Vietti, T.J.; Denman Hammond, G. Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer 1989, 64, 1082–1095. [Google Scholar] [CrossRef]
- Czauderna, P; Haeberle, E.; Hiyama, A.; Rangaswami, M.; Krailo, R.; Maibach, E.; Rinaldi, Y.; Feng, D.; Aronson, M.; Malogolowkin, K.; et al. The Children’s Hepatic Tumors International Collaboration (Chic): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur. J. Cancer 2016, 52, 92–101. [Google Scholar] [CrossRef]
- Luo, J.H.; Ren, B.; Keryanov, S.; Tseng, G.C.; Rao, U.N.; Monga, S.P.; Strom, S.; Demetris, A.J.; Nalesnik, M.; Ranganathan, S.; et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology 2006, 44, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Valanejad, L.; Lewis, K.; Wright, M.; Jiang, Y.; D’Souza, A.; Karns, R.; Sheridan, R.; Gupta, A.; Bove, K.; et al. Fxr-Gankyrin axis is involved in development of pediatric liver cancer. Carcinogenesis 2017, 38, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Cairo, S.; Armengol, C.; De Reyniès, A.; Wei, Y.; Thomas, E.; Renard, C.A.; Pontoglio, M.; Pontoglio, H.; Strick-Marchand, F.; Levillayer, Y.; et al. Hepatic stem-like phenotype and interplay of Wnt/Beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell 2008, 14, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Eichenmüller, M.; Trippel, F.; Kreuder, M.; Beck, A.; Schwarzmayr, T.; Häberle, B.; Kappler, R.; Cairo, S.; Leuschner, I.; Strom, T.M.; et al. The genomic landscape of hepatoblastoma and their progenies with Hcc-like features. J. Hepatol. 2014, 61, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; El Jabbour, T.; Ainechi, S.; Gay, L.M.; Elvin, J.A.; Vergilio, J.A.; Fabrizio, D.; Suh, J.; Ramkisson, S.H.; Ali, S.M.; et al. General paucity of genomic alteration and low tumor mutation burden in refractory and metastatic hepatoblastoma: Comprehensive genomic profiling study. Hum. Pathol. 2017, 70, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Curia, M.C.; Zuckermann, M.; De Lellis, L.; Catalano, T.; Lattanzio, R.; Aceto, G.; Mariani-Costantini, R.; Veschi, S.; Cama, A.; Otte, J.-B.; et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 Mutations. Mod. Pathol. 2008, 21, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Udatsu, Y.; Kusafuka, T.; Kuroda, S.; Miao, J.; Okada, A. High frequency of beta-catenin mutations in hepatoblastoma. Pediatr. Surg. Int. 2001, 17, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, H.; Horie, H.; Hiyama, E.; Matsunaga, T.; Hayashi, Y.; Watanabe, Y.; Ozaki, T.; Suita, S.; Kaneko, M.; Sasaki, F.; et al. Frequent deletions and mutations of the beta-catenin gene are associated with overexpression of cyclin D1 and fibronectin and poorly differentiated histology in childhood hepatoblastoma. Clin. Cancer Res. 2001, 7, 901–908. [Google Scholar]
- Koch, A.; Denkhaus, D.; Albrecht, S.; Leuschner, I.; von Schweinitz, D.; Pietsch, T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999, 59, 269–273. [Google Scholar]
- Bläker, H.; Hofmann, W.J.; Rieker, R.J.; Penzel, R.; Graf, M.; Otto, H.F. Beta-catenin accumulation and mutation of the CTNNB1 gene in hepatoblastoma. Genes Chromosomes Cancer 1999, 25, 399–402. [Google Scholar] [CrossRef]
- Weber, R.G.; Pietsch, T.; von Schweinitz, D.; Lichter, P. Characterization of genomic alterations in hepatoblastomas. A role for gains on chromosomes 8q and 20 as predictors of poor outcome. Am. J. Pathol. 2000, 157, 571–578. [Google Scholar] [CrossRef]
- Park, W.S.; Oh, R.R.; Park, J.Y.; Kim, P.J.; Shin, M.S.; Lee, J.H.; Kim, H.S.; Lee, S.H.; Kim, S.Y.; et al. Nuclear localization of beta-catenin is an important prognostic factor in hepatoblastoma. J. Pathol. 2001, 193, 483–490. [Google Scholar] [CrossRef]
- Crippa, S.; Ancey, P.B.; Vazquez, J.; Angelino, P.; Rougemont, A.L.; Guettier, C.; Meylan, E.; Zoete, V.; Delorenzi, M.; Michielin, O. Mutant CTNNB1 and histological heterogeneity define metabolic subtypes of hepatoblastoma. EMBO Mol. Med. 2017, 9, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Comerford, S.A.; Hinnant, E.A.; Chen, Y.; Bansal, H.; Klapproth, S.; Rakheja, D.; Hammer, R.E.; Finegold, M.J.; Lopez-Terrada, D.; O’Donnell, K.A.; et al. Hepatoblastoma modeling in mice places Nrf2 within a cancer field established by mutant beta-catenin. JCI Insight 2016, 1, e88549. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xi, Y.; Chen, T.; Wang, J.Y.; Tao, D.L.; Wu, Z.L.; Li, L.; Li, Y.-P.; Li, C.; Zeng, R. Caprin-2 enhances canonical Wnt signaling through regulating Lrp5/6 phosphorylation. J. Cell Biol. 2008, 182, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Dong, R.; Jing, Y.; Xu, D.; Wang, Q.; Chen, L.; Liu, L.; Li, Q.; Huang, Y.; Zhang, Y.; et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 2014, 60, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Cast, A.; Valanejad, L.; Wright, M.; Nguyen, P.; Gupta, A.; Zhu, L.; Timchenko, N.; Shin, S. C/Ebpalpha-dependent preneoplastic tumor foci are the origin of hepatocellular carcinoma and aggressive pediatric liver cancer. Hepatology 2018, 67, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Ruck, P.; Xiao, J.C. Stem-like cells in hepatoblastoma. Med. Pediatr. Oncol. 2002, 39, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lin, M.; Jiang, X.; Ye, J.; Guo, T.; Shi, Y.; Bian, X. The recent advances on liver cancer stem cells: Biomarkers, separation, and therapy. Anal. Cell Pathol. 2017, 2017, 5108653. [Google Scholar] [CrossRef] [PubMed]
- Bahnassy, A.A.; Fawzy, M.; El-Wakil, M.; Zekri, A.R.N.; Abdel-Sayed, A.; Sheta, M. Aberrant expression of cancer stem cell markers (Cd44, Cd90, and Cd133) contributes to disease progression and reduced survival in hepatoblastoma patients: 4-year survival data. Transl. Res. 2015, 165, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, K.H.; Chan, Y.P.; Lee, T.K.; Kwan, P.S.; Castilho, A.; Zheng, B.J.; Ng, I.; Man, K.; Zheng, B.-J.; et al. Mir-130b promotes Cd133+ liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010, 7, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Biology and clinical implications of Cd133+ liver cancer stem cells. Exp. Cell Res. 2013, 319, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rountree, C.B.; Senadheera, S.; Mato, J.M.; Crooks, G.M.; Lu, S.C. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology 2008, 47, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Rountree, C.B.; Ding, W.; He, L.; Stiles, B. Expansion of Cd133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem Cells 2009, 27, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; James, D.; Utley, S.; Cu, N.; Coblens, O.; Mak, K.; Wang, K.S.; Rountree, B.; Kahn, M. Fibroblast growth factor receptor-mediated activation of akt-beta-catenin-cbp pathway regulates survival and proliferation of murine hepatoblasts and hepatic tumor initiating stem cells. PLoS ONE 2012, 7, e50401. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; James, D.; Shivakumar, P.; Nguyen, M.V.; Utley, S.; Mak, K.; Groff, M.; Wu, A.; Zhou, S.; Wang, L.; et al. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 2014, 60, 941–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.C.; Yang, J.Y.; Yan, L.N. Relevant markers of cancer stem cells indicate a poor prognosis in hepatocellular carcinoma patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1007–1016. [Google Scholar] [CrossRef]
- Tang, K.H.; Ma, S.; Lee, T.K.; Chan, Y.P.; Kwan, P.S.; Tong, C.M.; Lo, C.M.; Ng, I.O.; Man, K.; To, K.-F.; et al. Cd133+ Liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/Cxcl1 signaling. Hepatology 2012, 55, 807–820. [Google Scholar] [CrossRef]
- Tang, Y.; Berlind, J.; Mavila, N. Inhibition of CREB binding protein-beta-catenin signaling down regulates Cd133 expression and activates PP2A-PTEN signaling in tumor initiating liver cancer cells. Cell Commun. Signal. 2018, 16, 9. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.J.; Chan, K.W.; Guan, X.Y. Cd133+ HCC Cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef]
- Stafman, L.L.; Williams, A.P.; Garner, E.F.; Aye, J.M.; Stewart, J.E.; Yoon, K.J.; Beierle, E.A.; Whelan, K. Targeting Pim Kinases Affects Maintenance of Cd133 Tumor Cell Population in Hepatoblastoma. Transl. Oncol. 2019, 12, 200–208. [Google Scholar] [CrossRef]
- Yamashita, T.; Budhu, A.; Forgues, M.; Wang, X.W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007, 67, 10831–10839. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Gerasimidou, D.; Kuwahara, R.; Hytiroglou, P.; Yoo, J.E.; Park, Y.N.; Theise, N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011, 53, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Theise, N.D.; Schmelzer, E.; Boulter, L.; Gires, O.; van Grunsven, L.A. EpCAM and the biology of hepatic stem/progenitor cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G233–G250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Okabe, M.; Suzuki, K.; Kamiya, Y.; Tsukahara, Y.; Saito, S.; Miyajima, A. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: Drastic change of EpCAM expression during liver development. Mech. Dev. 2009, 126, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Gires, O.; Kieu, C.; Papior, P.; Baeuerle, P.A.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Yamashita, T.; Forgues, M.; Wang, W.; Kim, J.W.; Ye, Q.; Jia, H.; Tang, Z.Y.; Budhu, A.; Zanetti, K.A.; Chen, Y.; et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008, 68, 1451–1461. [Google Scholar] [CrossRef]
- Münz, M.; Murr, A.; Kvesic, M.; Rau, D.; Mangold, S.; Pflanz, S.; Rüttinger, D.; Lumsden, J.; Volkland, J.; Fagerberg, J.; et al. Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies. Cancer Cell Int. 2010, 10, 44. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Fan, S.T.; Chu, P.W.; Lam, C.T.; Poon, R.T.P. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J.E.M.T. Cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Campbell, L.L.; Brooks, M.; Reinhard, F.; Zhang, C.C.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Ansieau, S.; Hinkal, G.; Thomas, C.; Bastid, J.; Puisieux, A. Early origin of cancer metastases: Dissemination and evolution of premalignant cells. Cell Cycle 2008, 7, 3659–3663. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tian, X.; Gong, W.; Sun, B.; Li, G.; Liu, D.; Song, T.; Guo, P.; He, Y.; Chen, Z.; et al. Periostin mediates epithelial-mesenchymal transition through the MAPK/ERK pathway in hepatoblastoma. Cancer Biol. Med. 2019, 16, 89–100. [Google Scholar]
- Chen, C.; Liang, Q.Y.; Chen, H.K.; Wu, P.F.; Feng, Z.Y.; Ma, X.M.; Zhou, G.Q.; Wu, H.R. Dram1 regulates the migration and invasion of hepatoblastoma cells via autophagy-EMT pathway. Oncol. Lett. 2018, 16, 2427–2433. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Yang, F.; Liu, Y. Kockdown of Oip5-As1 Expression Inhibits Proliferation, Metastasis and Emt Progress in Hepatoblastoma Cells through up-Regulating Mir-186a-5p and Down-Regulating Zeb1. Biomed. Pharmacother. 2018, 101, 14–23. [Google Scholar] [CrossRef]
- Fu, X.; Cui, P.; Chen, F.; Xu, J.; Gong, L.; Jiang, L.; Xiao, Y.; Zhang, D. Thymosin beta 4 promotes hepatoblastoma metastasis via the induction of epithelial-mesenchymal transition. Mol. Med. Rep. 2015, 12, 127–132. [Google Scholar] [CrossRef]
- Monga, S.P. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef]
- Russell, J.O.; Monga, S.P. Wnt/Beta-catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef]
- Tamai, K.; Zeng, X.; Liu, C.; Zhang, X.; Harada, Y.; Chang, Z.; He, X.A. Mechanism for Wnt coreceptor activation. Mol. Cell 2004, 13, 149–156. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/Beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Tamai, K.; Semenov, M.; Kato, Y.; Spokony, R.; Liu, C.; Katsuyama, Y.; He, X.; Hess, F.; Saint-Jeannet, J.-P. Ldl-receptor-related proteins in wnt signal transduction. Nature 2000, 407, 530–535. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef]
- Purcell, R.; Childs, M.; Maibach, R.; Miles, C.; Turner, C.; Zimmermann, A.; Sullivan, M. Hgf/C-Met related activation of beta-catenin in hepatoblastoma. J. Exp. Clin. Cancer Res. 2011, 30, 96. [Google Scholar] [CrossRef]
- Koch, A.; Weber, N.; Waha, A.; Hartmann, W.; Denkhaus, D.; Behrens, J.; Pietsch, T.; Behrens, J.; Birchmeier, W.; von Schweinitz, D. Mutations and elevated transcriptional activity of conductin (AXIN2) in hepatoblastomas. J. Pathol. 2004, 204, 546–554. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Zheng, K.; Wang, H.; Yang, S.; Han, W.; Yang, H.; Yu, Y.; Yang, Y.; Geng, D.; et al. Whole-Genome Sequencing Identifies a Novel Variation of Was Gene Coordinating with Heterozygous Germline Mutation of Apc to Enhance Hepatoblastoma Oncogenesis. Front. Genet. 2018, 9, 668. [Google Scholar] [CrossRef]
- Tao, J.; Calvisi, D.F.; Ranganathan, S.; Cigliano, A.; Zhou, L.; Singh, S.; Ribback, S.; Jiang, L.; Fan, B.; Terracciano, L.; et al. Activation of beta-catenin and yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 2014, 3, 690–701. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef]
- Valanejad, L.; Cast, A.; Wright, M.; Bissig, K.D.; Karns, R.; Weirauch, M.T.; Timchenko, N. PARP1 activation increases expression of modified tumor suppressors and pathways underlying development of aggressive hepatoblastoma. Commun. Biol. 2018, 1, 67. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamamichi, T.; Shinzawa, K.; Kasahara, Y.; Nojima, S.; Kodama, T.; Kikuchi, A.; Obika, S.; Takehara, T.; Morri, E.; et al. Greb1 induced by Wnt signaling promotes development of hepatoblastoma by suppressing TGFbeta signaling. Nat. Commun. 2019, 10, 3882. [Google Scholar] [CrossRef]
- Aretz, S.; Koch, A.; Uhlhaas, S.; Friedl, W.; Propping, P.; Schweinitz, D.V.; Pietsch, T. Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr. Blood Cancer 2006, 47, 811–818. [Google Scholar] [CrossRef]
- Mokkapati, S.; Niopek, K.; Huang, L.; Cunniff, K.J.; Ruteshouser, E.C.; DeCaestecker, M.; Huff, V.; Finegold, M.J. Beta-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma. Cancer Res. 2014, 74, 4515–4525. [Google Scholar] [CrossRef]
- Yang, W.; Yan, H.X.; Chen, L.; Liu, Q.; He, Y.Q.; Yu, L.X.; Chen, C.; Zhang, S.H.; Huang, D.D.; Tang, L.; et al. Wnt/Beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008, 68, 4287–4295. [Google Scholar] [CrossRef]
- Cristóbal, I.; Sanz-Álvarez, M.; Luque, M.; Caramés, C.; Rojo, F.; García-Foncillas, J. The Role of Micrornas in Hepatoblastoma Tumors. Cancers 2019, 11, 409. [Google Scholar] [CrossRef]
- Lou, W.; Liu, J.; Gao, Y.; Zhong, G.; Ding, B.; Xu, L.; Fan, W. MicroRNA regulation of liver cancer stem cells. Am. J. Cancer Res. 2018, 8, 1126–1141. [Google Scholar]
- Cairo, S.; Wang, Y.; de Reyniès, A.; Duroure, K.; Dahan, J.; Redon, M.J.; Buendia, M.A.; Fabre, M.; McClelland, M.; Wang, X.W.; et al. Stem cell-like micro-RNA signature driven by Myc in aggressive liver cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 20471–20476. [Google Scholar] [CrossRef] [Green Version]
- Indersie, E.; Lesjean, S.; Hooks, K.B.; Sagliocco, F.; Ernault, T.; Cairo, S.; Grotzer, M.; Rullier, A.; Taque, S.; Guettier, C.; et al. MicroRNA therapy inhibits hepatoblastoma growth in vivo by targeting beta-catenin and Wnt signaling. Hepatol. Commun. 2017, 1, 168–183. [Google Scholar] [CrossRef]
- Cai, H.; Chen, Y.; Yang, X.; Ma, S.; Wang, Q.; Zhang, Y.; Yuan, Y.; Niu, X.; Ding, G. Let7b Modulates the Wnt/Beta-catenin pathway in liver cancer cells via downregulated Frizzled4. Tumour Biol. 2017, 39, 1010428317716076. [Google Scholar] [CrossRef]
- Jin, B.; Wang, W.; Meng, X.X.; Du, G.; Li, J.; Zhang, S.Z.; Fu, Z.H.; Zhou, B.H. Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway. BMC Cancer 2016, 16, 863. [Google Scholar] [CrossRef]
- Chai, S.; Ng, K.Y.; Tong, M.; Lau, E.Y.; Lee, T.K.; Chan, K.W.; Wong, N.; Yuan, Y.F.; Cheung, S.T.; Wang, X.Q.; et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/beta-catenin activation in liver cancer stem cells. Hepatology 2016, 64, 2062–2076. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, M.; Xie, X.; Huang, G.; Peng, Y.; Ren, D.; Kuang, M.; Lin, M.; Liu, B.; Liu, M.; et al. miR-217 targeting DKK1 promotes cancer stem cell properties via activation of the Wnt signaling pathway in hepatocellular carcinoma. Oncol. Rep. 2017, 38, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Tao, Z.H.; Wen, D.; Wan, J.L.; Liu, D.L.; Zhang, S.; Fan, J.; Cui, J.F.; Sun, H.C.; Wang, L.; et al. miR-612 suppresses the stemness of liver cancer via Wnt/Beta-catenin signaling. Biochem. Biophys. Res. Commun. 2014, 447, 210–215. [Google Scholar] [CrossRef]
- Xia, H.; Cheung, W.K.; Sze, J.; Lu, G.; Jiang, S.; Yao, H.; Lin, M.C.; Bian, X.W.; Poon, W.S.; Kung, H. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J. Biol. Chem. 2010, 285, 36995–37004. [Google Scholar] [CrossRef]
- Del Vecchio, C.A.; Feng, Y.; Sokol, E.S.; Tillman, E.J.; Sanduja, S.; Reinhardt, F.; Gupta, P.B. De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 2014, 12, e1001945. [Google Scholar] [CrossRef]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic plasticity: Driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Lenz, H.J.; Kahn, M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014, 105, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Delgado, E.R.; Yang, J.; So, J.; Fanti, M.; Leimgruber, S.; Kahn, M.; Monga, S.P.; Ishitani, T.; Shin, D.; Wilson, G.D. Identification and characterization of a novel small-molecule inhibitor of beta-catenin signaling. Am. J. Pathol. 2014, 184, 2111–2122. [Google Scholar] [CrossRef]
- McMillan, M.; Kahn, M. Investigating Wnt signaling: A chemogenomic safari. Drug Discov. Today 2005, 10, 1467–1474. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, S.H.; Moon, R.T.; Teo, J.L.; Kim, H.Y.; et al. A small molecule inhibitor of beta-catenin/creb-binding protein transcription [Corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef]
- Eguchi, M.; Nguyen, C.; Lee, S.C.; Kahn, M. Icg-001, a Novel Small Molecule Regulator of Tcf/Beta-Catenin Transcription. Med. Chem. 2005, 1, 467–472. [Google Scholar] [CrossRef]
- Handeli, S.; Simon, J.A. A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates Ppargamma and Ppardelta Activities. Mol. Cancer Ther. 2008, 7, 521–529. [Google Scholar] [CrossRef]
- Lepourcelet, M.; Chen, Y.N.P.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Shivdasani, R.A.; Bruseo, C.; Wood, A.W. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef]
- Wei, W.; Chua, M.S.; Grepper, S.; So, S. Small molecule antagonists of Tcf4/Beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int. J. Cancer 2010, 126, 2426–2436. [Google Scholar]
- Molina, L.; Yang, H.; Michael, A.O.A.; Oertel, M.; Bell, A.; Singh, S.; Monga, S.P.; Chen, X.; Tao, J. mTOR inhibition affects Yap1-Beta-catenin-induced hepatoblastoma growth and development. Oncotarget 2019, 10, 1475–1490. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavila, N.; Thundimadathil, J. The Emerging Roles of Cancer Stem Cells and Wnt/Beta-Catenin Signaling in Hepatoblastoma. Cancers 2019, 11, 1406. https://doi.org/10.3390/cancers11101406
Mavila N, Thundimadathil J. The Emerging Roles of Cancer Stem Cells and Wnt/Beta-Catenin Signaling in Hepatoblastoma. Cancers. 2019; 11(10):1406. https://doi.org/10.3390/cancers11101406
Chicago/Turabian StyleMavila, Nirmala, and Jyothi Thundimadathil. 2019. "The Emerging Roles of Cancer Stem Cells and Wnt/Beta-Catenin Signaling in Hepatoblastoma" Cancers 11, no. 10: 1406. https://doi.org/10.3390/cancers11101406



