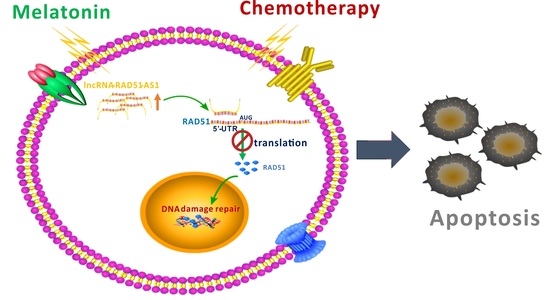
Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair
Abstract
1. Introduction
2. Results
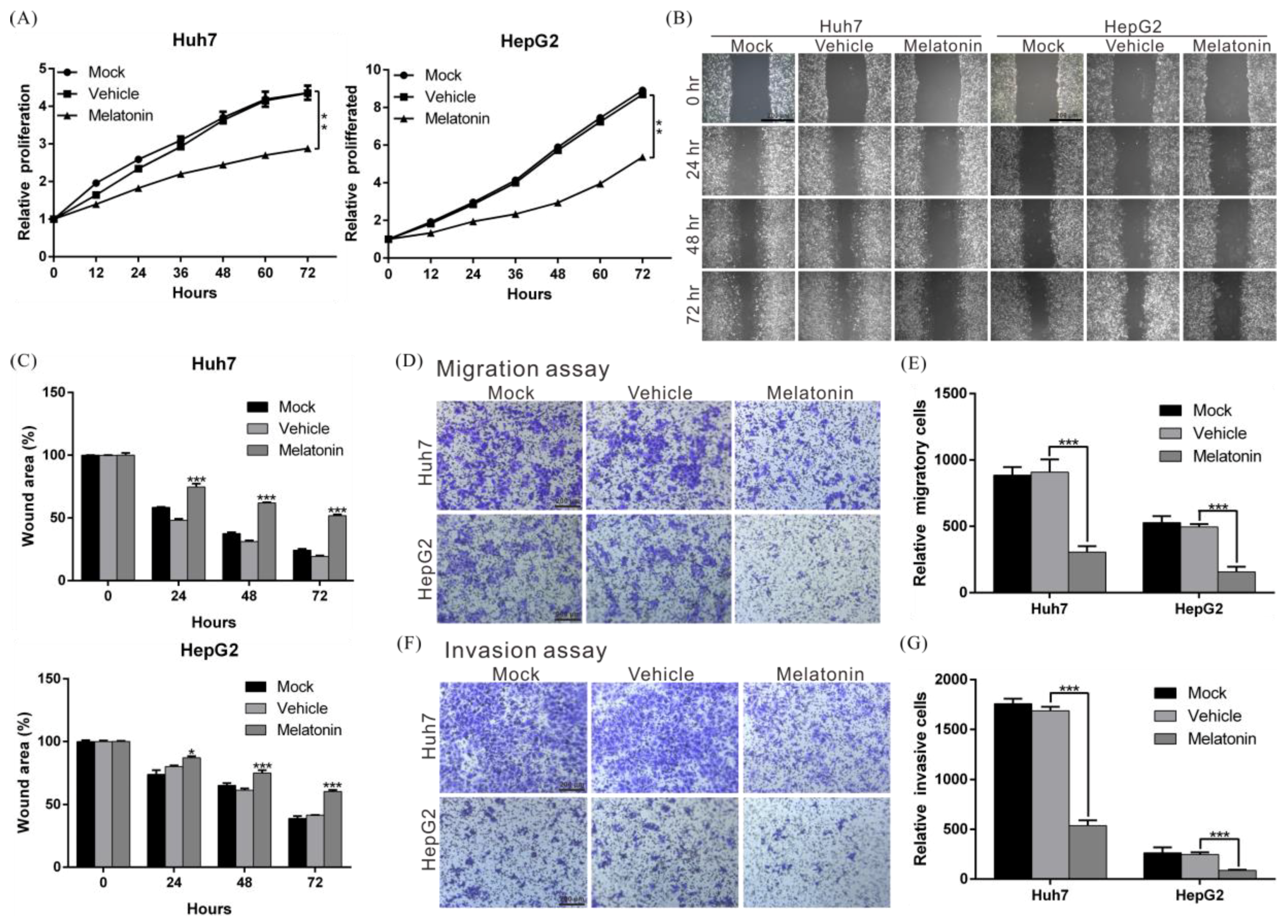
2.1. Melatonin Inhibits the Proliferation, Migration, and Invasion Abilities of HCC Cells
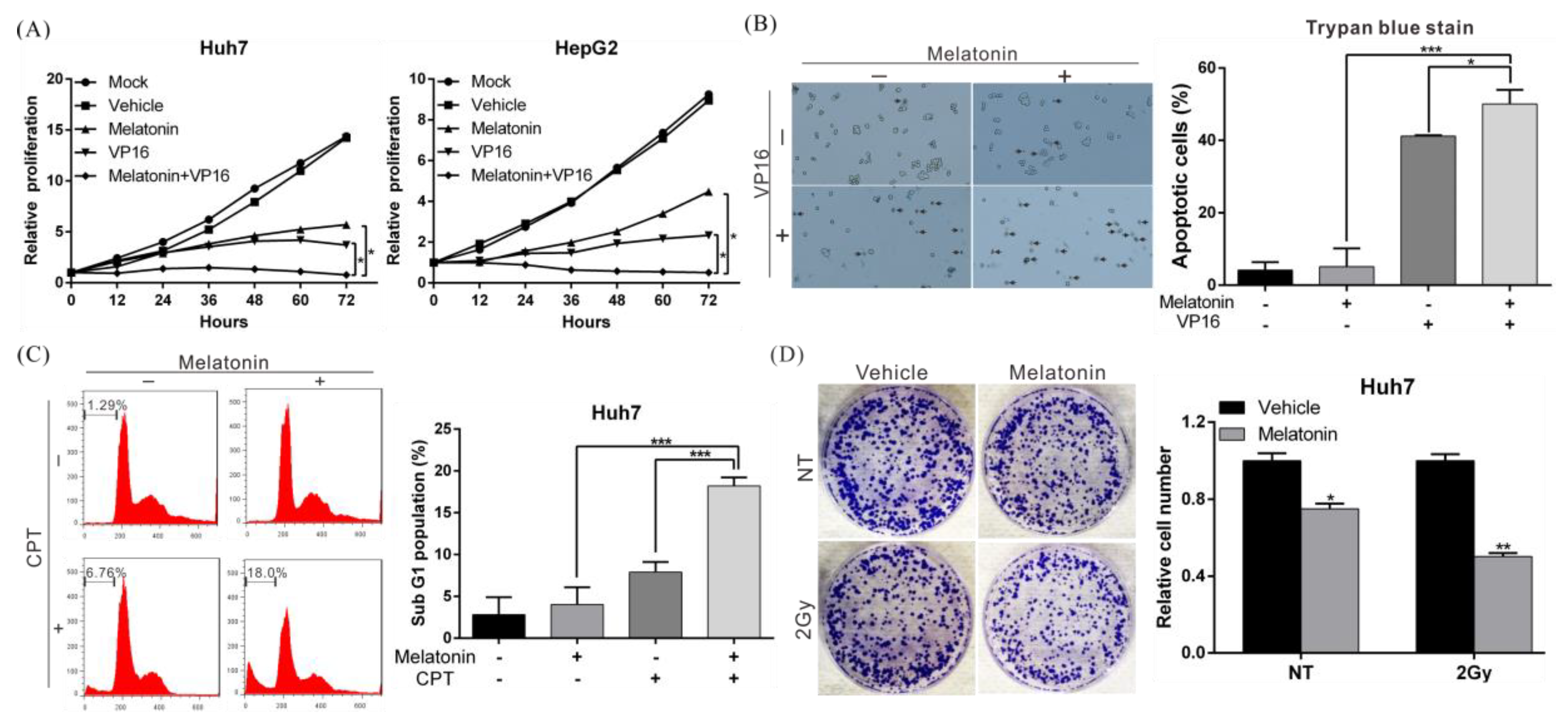
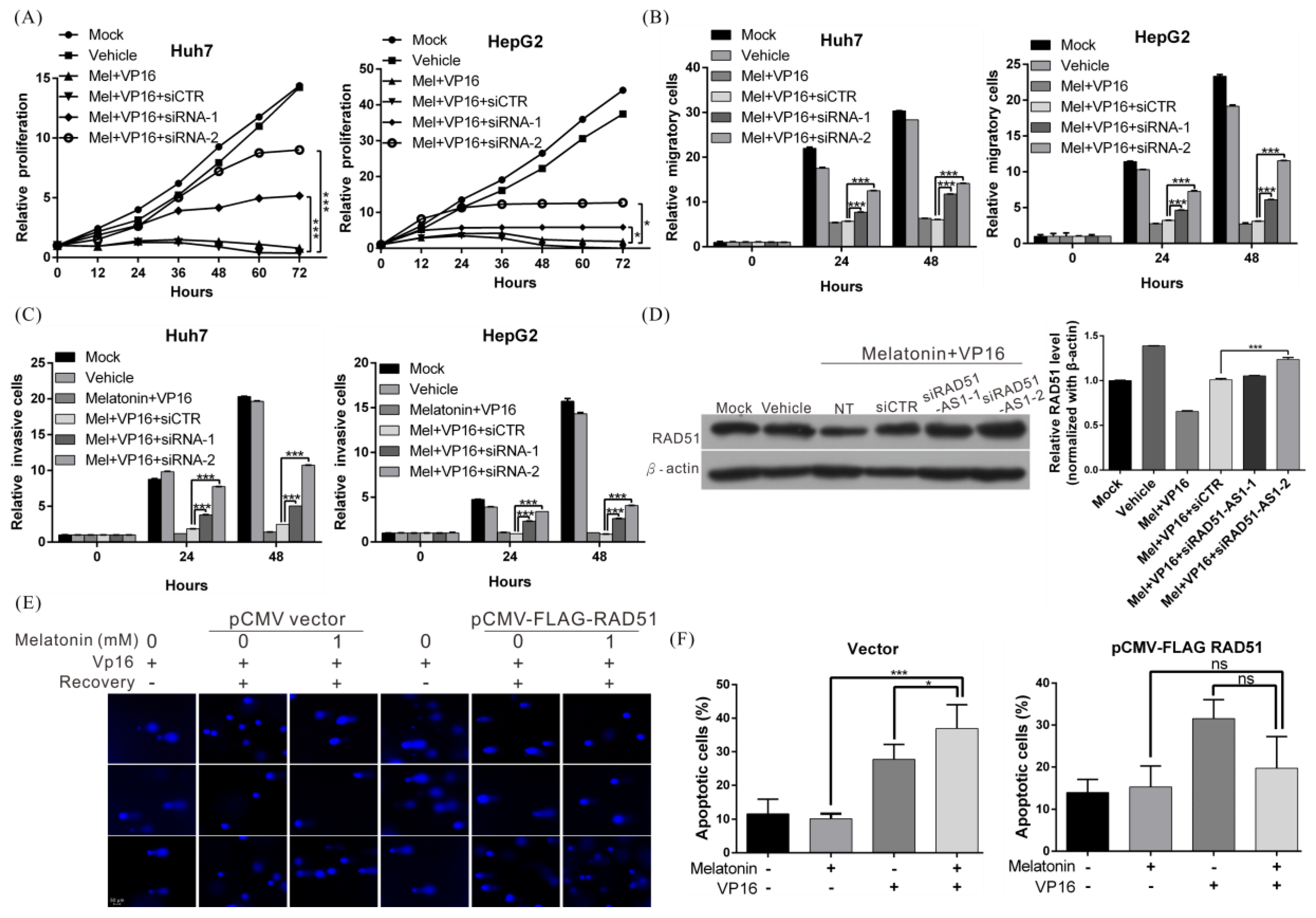
2.2. Melatonin Increases the Sensitivity of HCC Cells to Chemotherapy and Radiotherapy
2.3. Melatonin Inhibits the Growth of HCC Tumors and Increases the In Vivo Inhibitory Effects of Chemotherapeutic Drugs on Tumors
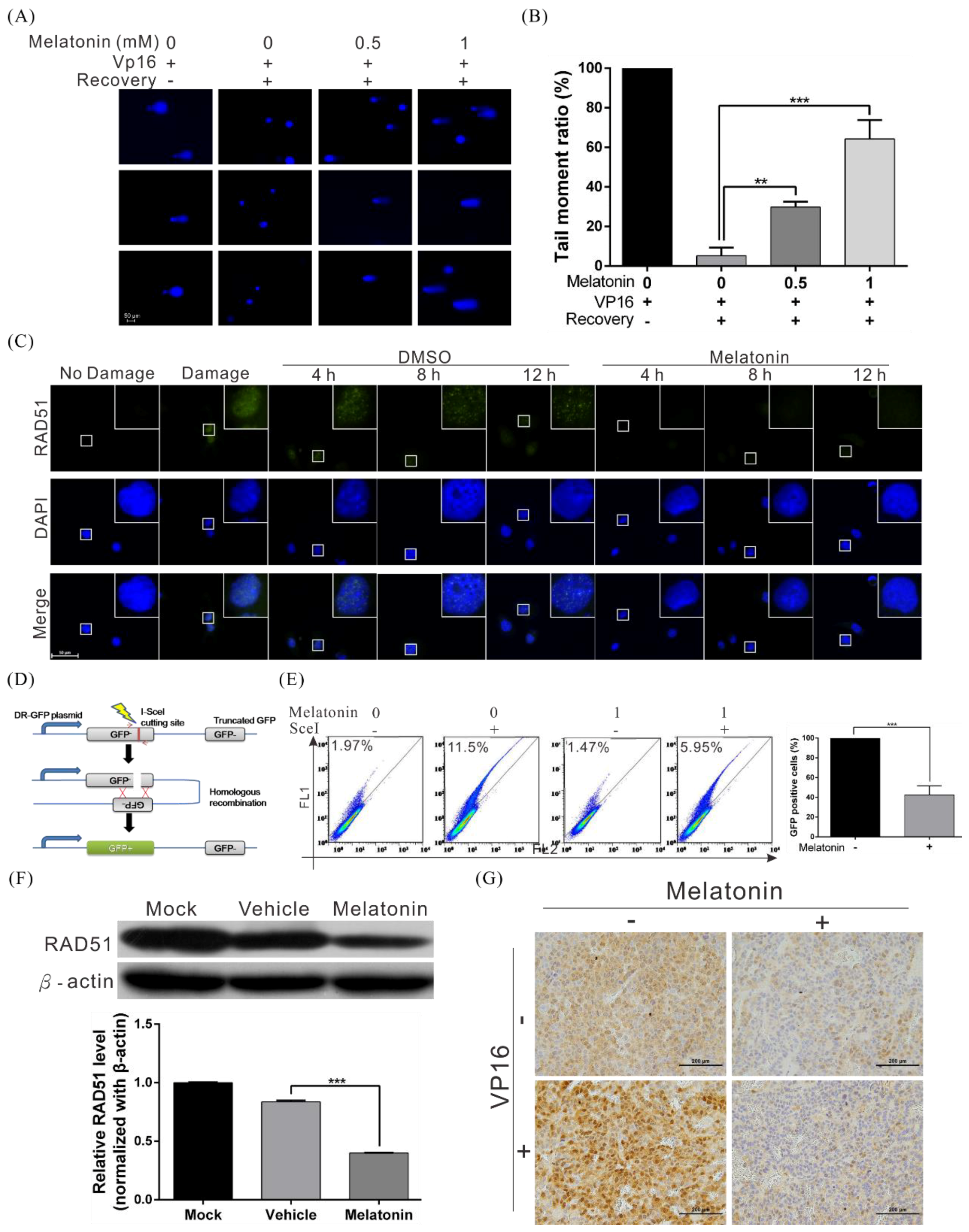
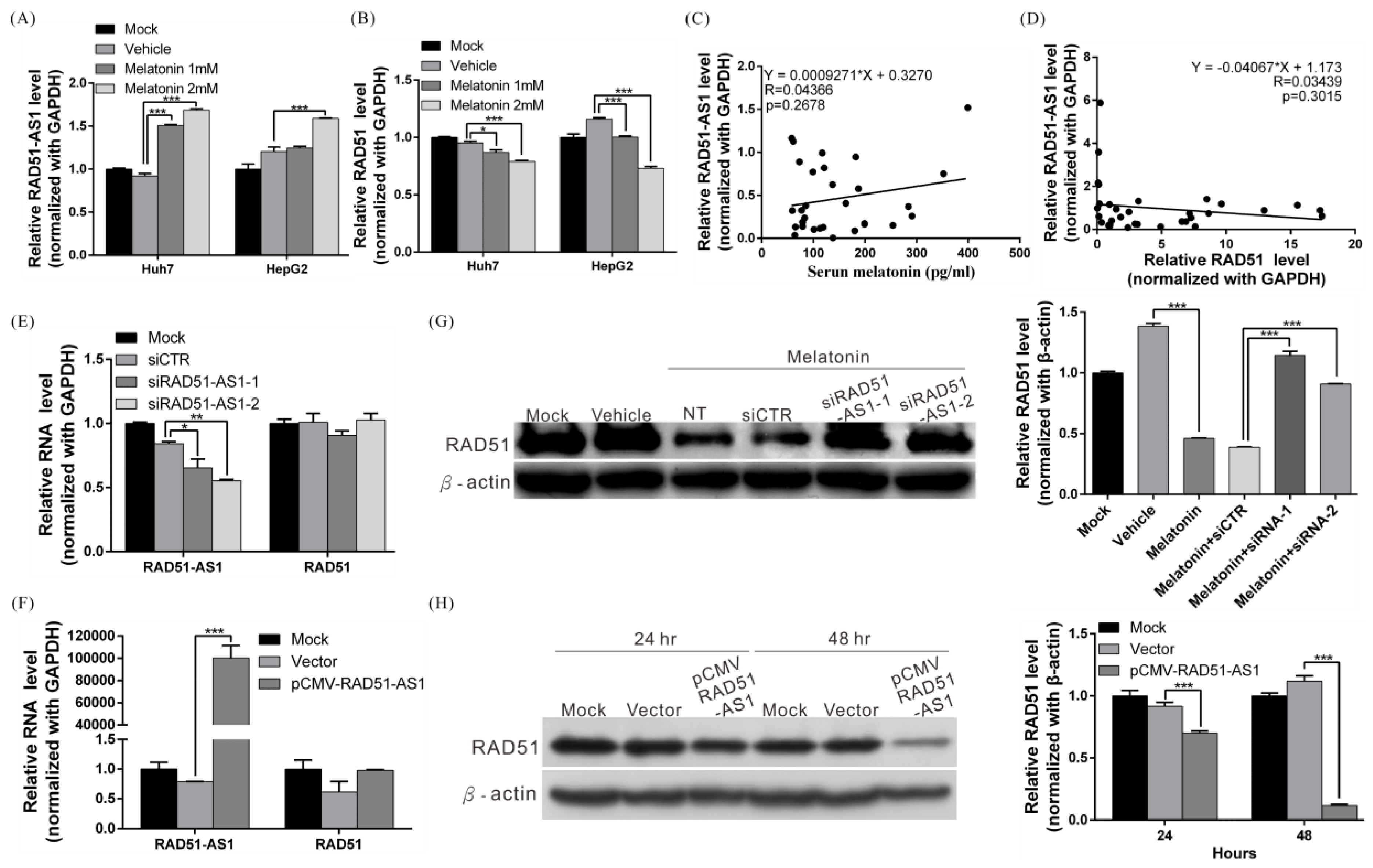
2.4. Melatonin Decreases the DNA Damage Repair Capacity of HCC Cells by Inhibiting RAD51 Expression
2.5. Melatonin Induces Expression of lncRNA RAD51-AS1, Causing Reduced RAD51 Levels
2.6. Melatonin Increases the Sensitivity of HCC Cells to Chemotherapeutic Agents by Inducing Expression of lncRNA RAD51-AS1
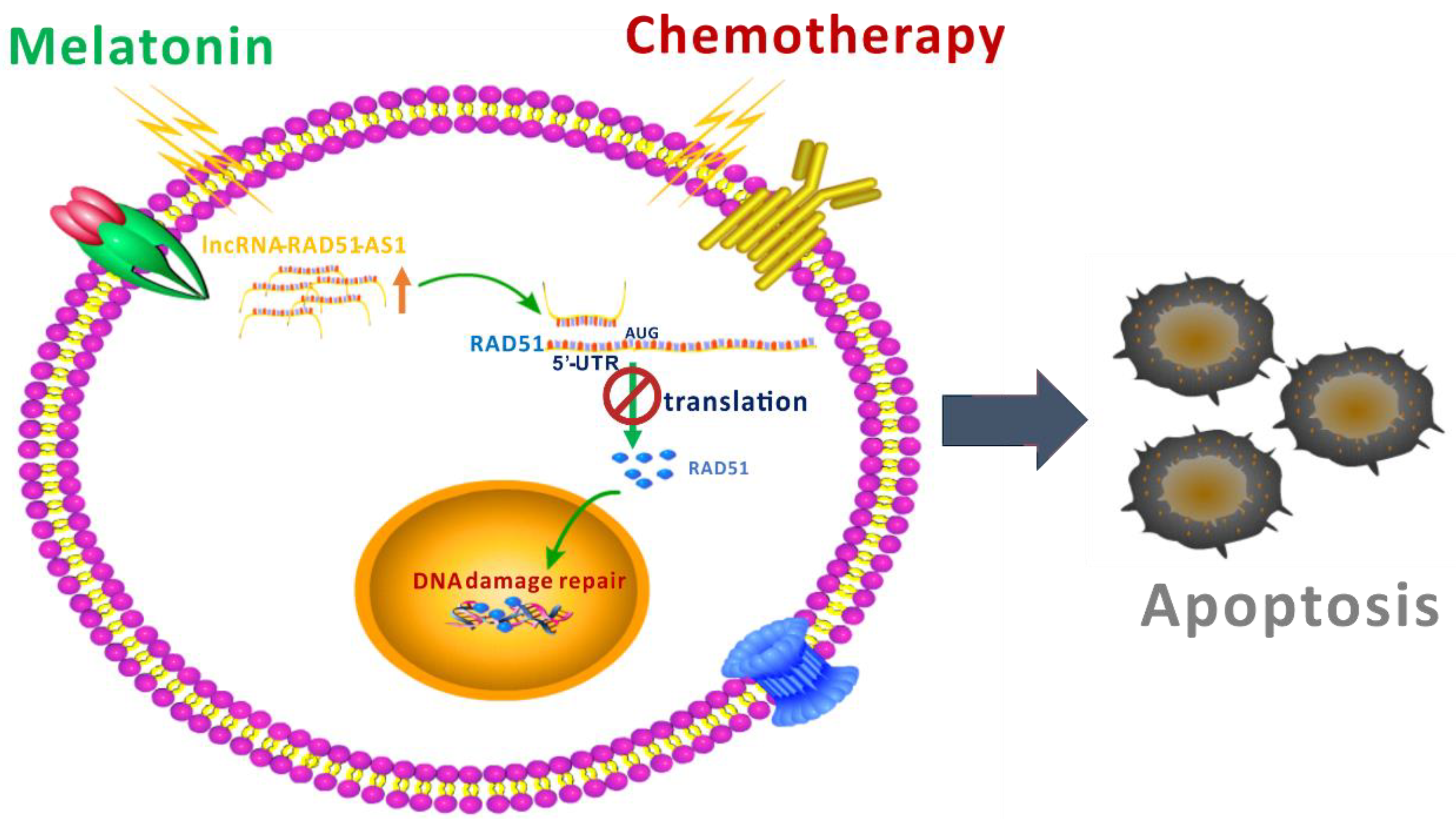
3. Discussion
4. Materials and Methods
4.1. Analysis of Melatonin, RAD51 and RAD51-AS1 Levels in Human Specimens
4.2. Cell Lines, Antibodies, Drug, siRNA and Plasmid Construction
4.3. Detection of lncRNA-RAD51-AS1 and RAD51 Levels Using Quantitative Real-Time RT-PCR
4.4. Transfection and Western Blotting Analysis
4.5. Cell Proliferation Assay
4.6. Cell Migration and Invasion Assays
4.7. Apoptosis Assay
4.8. Comet Assay
4.9. Immunofluorescence Staining of RAD51
4.10. HR Assay
4.11. Whole-Transcriptome Sequencing
4.12. Mice
4.13. Xenograft Assays and Drug Administration
4.14. Immunohistochemical Staining
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karaman, B.; Battal, B.; Sari, S.; Verim, S. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 18059–18060. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Wang, T.; Hu, X.; Hui, X.; Yan, M.; Gao, Q.; Chen, T.; Li, J.; Yao, M.; et al. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology 2011, 53, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Phan, H.; Yang, L.X. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012, 32, 1379–1386. [Google Scholar] [PubMed]
- Berte, N.; Piée-Staffa, A.; Piecha, N.; Wang, M.; Borgmann, K.; Kaina, B.; Nikolova, T. Targeting homologous recombination by pharmacological inhibitors enhances the killing response of glioblastoma cells treated with alkylating drugs. Mol. Cancer Ther. 2016, 15, 2665–2678. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Politis, T.; Darvesh, A.S. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010, 36, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, L.; Wu, G.; Konig, H.; Lin, X.; Li, G.; Qiu, X.L.; Chen, C.F.; Hu, C.M.; Goldblatt, E.; et al. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO Mol. Med. 2013, 5, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Dabin, J.; Fortuny, A.; Polo, S.E. Epigenome maintenance in response to DNA damage. Mol. Cell 2016, 62, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Her, C.; Chai, W. DNA excision repair at telomeres. DNA Repair (Amst.) 2015, 36, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008, 9, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Gao, Z.; Li, H.; Zhang, B.; Wang, G.; Zhang, Q.; Pei, D.; Zheng, J. DNA damage response--a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 2015, 358, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Iyama, T.; Wilson, D.M., 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst.) 2013, 12, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Krejci, L.; Altmannova, V.; Spirek, M.; Zhao, X. Homologous recombination and its regulation. Nucleic Acids Res. 2012, 40, 5795–5818. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Khanna, K.K.; Wiegmans, A.P. Targeting homologous recombination, new pre-clinical and clinical therapeutic combinations inhibiting RAD51. Cancer Treat Rev. 2015, 41, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, F.; Williams, K.P.; Smith, G.R.; Thomas, Z.; Allensworth, J.L.; Lyerly, H.K.; Diehl, A.M.; Morse, M.A.; Devi, G.R. Differential effects of arsenic trioxide on chemosensitization in human hepatic tumor and stellate cell lines. BMC Cancer 2012, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Kakadiya, R.; Su, T.L.; Lee, T.C. Combination of bifunctional alkylating agent and arsenic trioxide synergistically suppresses the growth of drug-resistant tumor cells. Neoplasia 2010, 12, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Stehle, J.H.; Saade, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebestény, T.; Maronde, E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res. 2011, 51, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Bartolomei, M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, S.; Fan, J.; Jin, Y.; Tian, B.; Zheng, X.; Fu, H. Nuclear retention of the lncRNA SNHG1 by doxorubicin attenuates hnRNPC-p53 protein interactions. EMBO Rep. 2017, 18, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Wood, J.; Haga, H.; Patel, T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014, 12, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, S.; Diederichs, S. From junk to master regulators of invasion: LncRNA functions in migration, EMT and metastasis. Int. J. Cancer 2016, 139, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Xiao, Y.F.; Tang, B.; Hu, C.J.; Xie, R.; Yang, S.M.; Li, B.S. Long noncoding RNA in digestive tract cancers: Function, mechanism, and potential biomarker. Oncologist 2015, 20, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Zheng, L.; Hu, Y.W.; Wang, Q. Characteristics of long non-coding RNA and its relation to hepatocellular carcinoma. Carcinogenesis 2014, 35, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.G.; Jensen, R.B.; Zagelbaum, J.; Rothenberg, E.; Morrical, S.W.; Wallace, S.S.; Sweasy, J.B. The tumor-associated variant RAD51 G151D induces a hyper-recombination phenotype. PLoS Genet. 2016, 12, e1006208. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D. DNA-repair gene mutations in metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 1803. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Lin, S.Y. DNA damage and breast cancer. World J. Clin. Oncol. 2011, 2, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Broustas, C.G.; Lieberman, H.B. DNA damage response genes and the development of cancer metastasis. Radiat. Res. 2014, 181, 111–130. [Google Scholar] [CrossRef] [PubMed]
- McNeil, E.M.; Melton, D.W. DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy. Nucleic Acids Res. 2012, 40, 9990–10004. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, T.; Senft, J.; Wang, Y.; Konat, G.W.; Wenger, S.L.; Reed, E.; Wang, W. BRCA1 contributes to cell cycle arrest and chemoresistance in response to the anticancer agent irofulven. Mol. Pharmacol. 2007, 71, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- McNeil, E.M.; Astell, K.R.; Ritchie, A.M.; Shave, S.; Houston, D.R.; Bakrania, P.; Jones, H.M.; Khurana, P.; Wallace, C.; Chapman, T.; et al. Inhibition of the ERCC1-XPF structure-specific endonuclease to overcome cancer chemoresistance. DNA Repair (Amst.) 2015, 31, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mohni, K.N.; Kavanaugh, G.M.; Cortez, D. ATR pathway inhibition is synthetically lethal in cancer cells with ERCC1 deficiency. Cancer Res. 2014, 74, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; O’Carrigan, B.; Jackson, S.P.; Yap, T.A. Targeting DNA repair in cancer: Beyond PARP inhibitors. Cancer Discov. 2017, 7, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Javle, M. DNA repair dysfunction in pancreatic cancer: A clinically relevant subtype for drug development. J. Natl. Compr. Cancer Netw. 2017, 15, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Qiu, J.; Zhang, N.; Ding, J.; Hua, K. E2F1-regulated long non-coding RNA RAD51-AS1 promotes cell cycle progression, inhibits apoptosis and predicts poor prognosis in epithelial ovarian cancer. Sci. Rep. 2017, 7, 4469. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, C.Y.; Ueng, S.H.; Hsueh, C.; Yeh, C.T.; Ho, J.Y.; Chou, L.F.; Wang, T.H. Corylin increases the sensitivity of hepatocellular carcinoma cells to chemotherapy through long noncoding RNA RAD51-AS1-mediated inhibition of DNA repair. Cell Death Dis. 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Blin-Wakkach, C.; Lezot, F.; Ghoul-Mazgar, S.; Hotton, D.; Monteiro, S.; Teillaud, C.; Pibouin, L.; Orestes-Cardoso, S.; Papagerakis, P.; Macdougall, M.; et al. Endogenous Msx1 antisense transcript: In vivo and in vitro evidences, structure, and potential involvement in skeleton development in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 7336–7341. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Meary, F.; Pibouin, L.; Jeanny, J.C.; Fernandes, I.; Poliard, A.; Hotton, D.; Berdal, A.; Babajko, S. Autoregulatory loop of Msx1 expression involving its antisense transcripts. J. Cell Physiol. 2009, 220, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Coudert, A.E.; Pibouin, L.; Vi-Fane, B.; Thomas, B.L.; Macdougall, M.; Choudhury, A.; Robert, B.; Sharpe, P.T.; Berdal, A.; Lezot, F. Expression and regulation of the Msx1 natural antisense transcript during development. Nucleic Acids Res. 2005, 33, 5208–5218. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Simons, R.W. The IS10 antisense RNA blocks ribosome binding at the transposase translation initiation site. EMBO J. 1990, 9, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille, F.; Unoson, C.; Vogel, J.; Wagner, E.G. An antisense RNA inhibits translation by competing with standby ribosomes. Mol. Cell 2007, 26, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.X.; Tao, Q.F.; Wang, J.; Yang, F.; Liu, L.; Wang, L.L.; Zhang, J.; Yang, Y.; Liu, H.; Wang, F.; et al. Antisense long non-coding RNA PCNA-AS1 promotes tumor growth by regulating proliferating cell nuclear antigen in hepatocellular carcinoma. Cancer Lett. 2014, 349, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kachhap, S.K.; Rosmus, N.; Collis, S.J.; Kortenhorst, M.S.; Wissing, M.D.; Hedayati, M.; Shabbeer, S.; Mendonca, J.; Deangelis, J.; Marchionni, L.; et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PLoS ONE 2010, 5, e11208. [Google Scholar] [CrossRef] [PubMed]
- Arias-Lopez, C.; Lazaro-Trueba, I.; Kerr, P.; Lord, C.J.; Dexter, T.; Iravani, M.; Ashworth, A.; Silva, A. p53 modulates homologous recombination by transcriptional regulation of the RAD51 gene. EMBO Rep. 2006, 7, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Alonso-González, C.; González, A.; Martínez-Campa, C.; Gómez-Arozamena, J.; Cos, S. Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double-strand DNA break repair. J. Pineal Res. 2015, 58, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.A.; Moradi Marjaneh, M.; Al-Ejeh, F.; Lim, Y.C.; Shi, W.; Sivakumaran, H.; Tropée, R.; Patch, A.M.; Clark, M.B.; Bartonicek, N.; et al. Long noncoding RNAs CUPID1 and CUPID2 mediate breast cancer risk at 11q13 by modulating the response to DNA damage. Am. J. Hum. Genet. 2017, 101, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Mathur, R.; Hu, X.; Liu, Y.; Zhang, X.; Peng, G.; Lu, X. Long non-coding RNA ANRIL (CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cell Signal. 2013, 25, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Liu, Y.; Han, C.; Zhang, X.; Lu, X. Noncoding RNAs in DNA repair and genome integrity. Antioxid. Redox Signal. 2014, 20, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Wu, C.H.; Yeh, C.T.; Su, S.C.; Hsia, S.M.; Liang, K.H.; Chen, C.C.; Hsueh, C.; Chen, C.Y. Melatonin suppresses hepatocellular carcinoma progression via lncRNA-CPS1-IT-mediated HIF-1alpha inactivation. Oncotarget 2017, 8, 82280–82293. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.H.; Moloudizargari, M.; Ghobadi, E.; Fallah, M.; Abdollahi, M. Melatonin as a multifunctional anti-cancer molecule: Implications in gastric cancer. Life Sci. 2017, 185, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Borin, T.F.; Arbab, A.S.; Gelaleti, G.B.; Ferreira, L.C.; Moschetta, M.G.; Jardim-Perassi, B.V.; Iskander, A.S.; Varma, N.R.; Shankar, A.; Coimbra, V.B.; et al. Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J. Pineal Res. 2016, 60, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Lin, C.W.; Chien, M.H.; Reiter, R.J.; Su, S.C.; Hsieh, Y.H.; Yang, S.F. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J. Pineal Res. 2016, 61, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Lin, Y.S.; Chen, Y.; Yeh, C.T.; Huang, Y.L.; Hsieh, T.H.; Shieh, T.M.; Hsueh, C.; Chen, T.C. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget 2015, 6, 23342–23357. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Chang, J.L.; Ho, J.Y.; Wu, H.C.; Chen, T.C. EphrinA5 suppresses colon cancer development by negatively regulating epidermal growth factor receptor stability. FEBS J. 2012, 279, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.C.; Shieh, T.M.; Hsueh, C.; Wang, S.H.; Leu, Y.L.; Lian, J.H.; Wang, T.H. Corylin suppresses hepatocellular carcinoma progression via the inhibition of epithelial-mesenchymal transition, mediated by long noncoding RNA GAS5. Int. J. Mol. Sci. 2018, 19, E380. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Chen, C.-Y.; Wang, S.-H.; Yeh, C.-T.; Su, S.-C.; Ueng, S.-H.; Chuang, W.-Y.; Hsueh, C.; Wang, T.-H. Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers 2018, 10, 320. https://doi.org/10.3390/cancers10090320
Chen C-C, Chen C-Y, Wang S-H, Yeh C-T, Su S-C, Ueng S-H, Chuang W-Y, Hsueh C, Wang T-H. Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers. 2018; 10(9):320. https://doi.org/10.3390/cancers10090320
Chicago/Turabian StyleChen, Chin-Chuan, Chi-Yuan Chen, Shu-Huei Wang, Chau-Ting Yeh, Shih-Chi Su, Shir-Hwa Ueng, Wen-Yu Chuang, Chuen Hsueh, and Tong-Hong Wang. 2018. "Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair" Cancers 10, no. 9: 320. https://doi.org/10.3390/cancers10090320
APA StyleChen, C.-C., Chen, C.-Y., Wang, S.-H., Yeh, C.-T., Su, S.-C., Ueng, S.-H., Chuang, W.-Y., Hsueh, C., & Wang, T.-H. (2018). Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers, 10(9), 320. https://doi.org/10.3390/cancers10090320