Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System
Abstract
:1. Introduction
2. Results
2.1. Patient Population
2.2. Patient and Tumor Characteristics by TRG Grouping
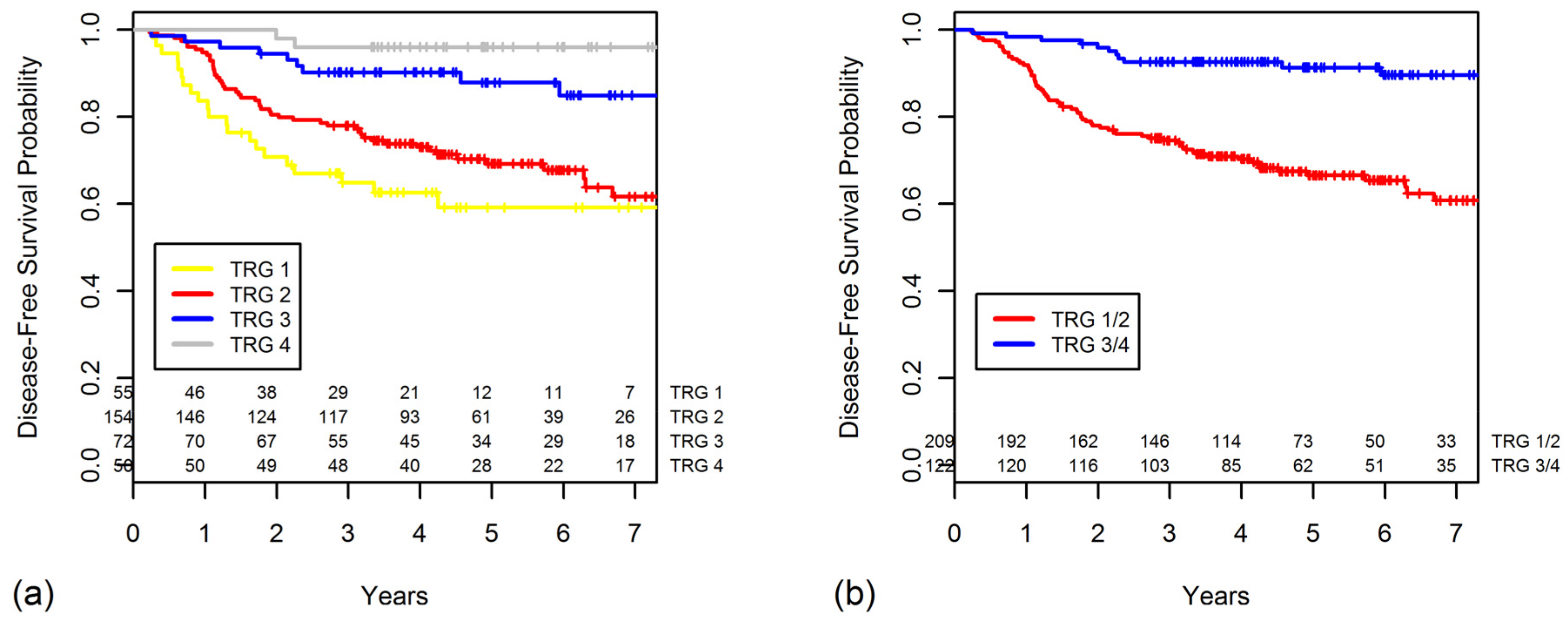
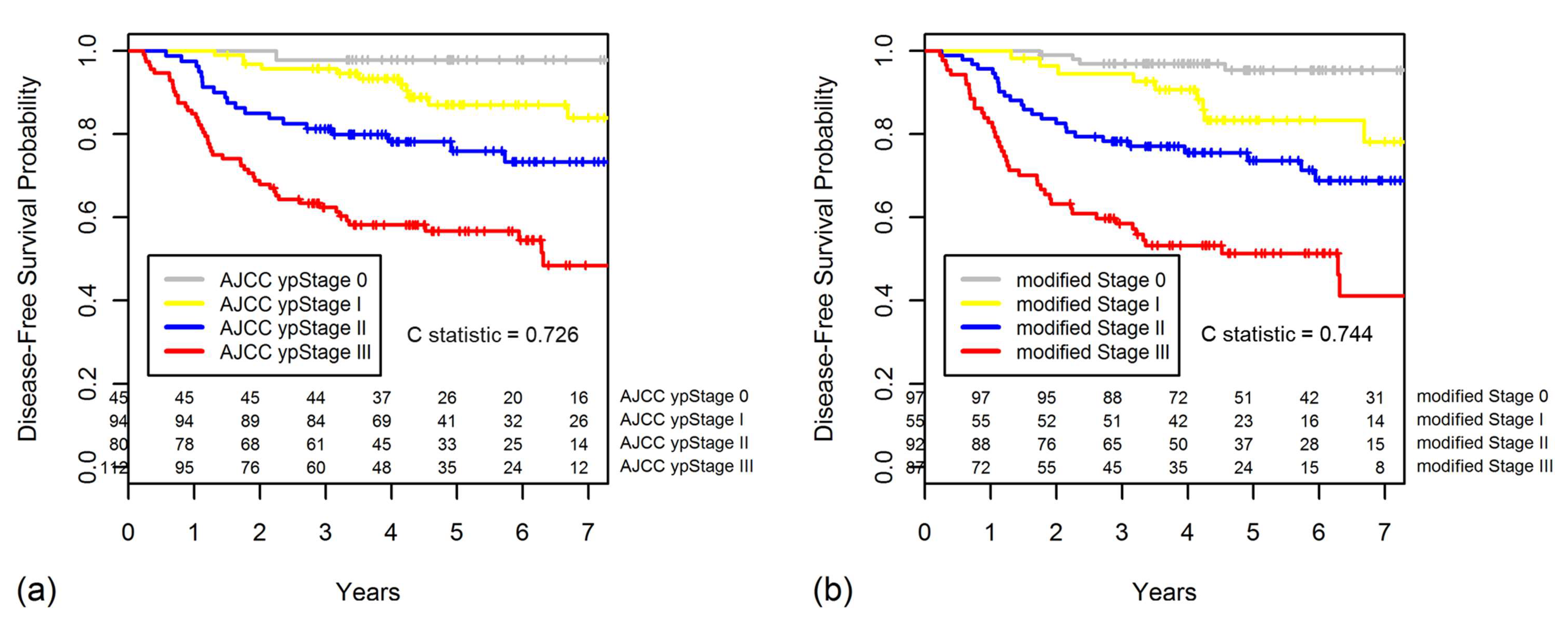
2.3. TRG as a Prognostic Factor for DFS
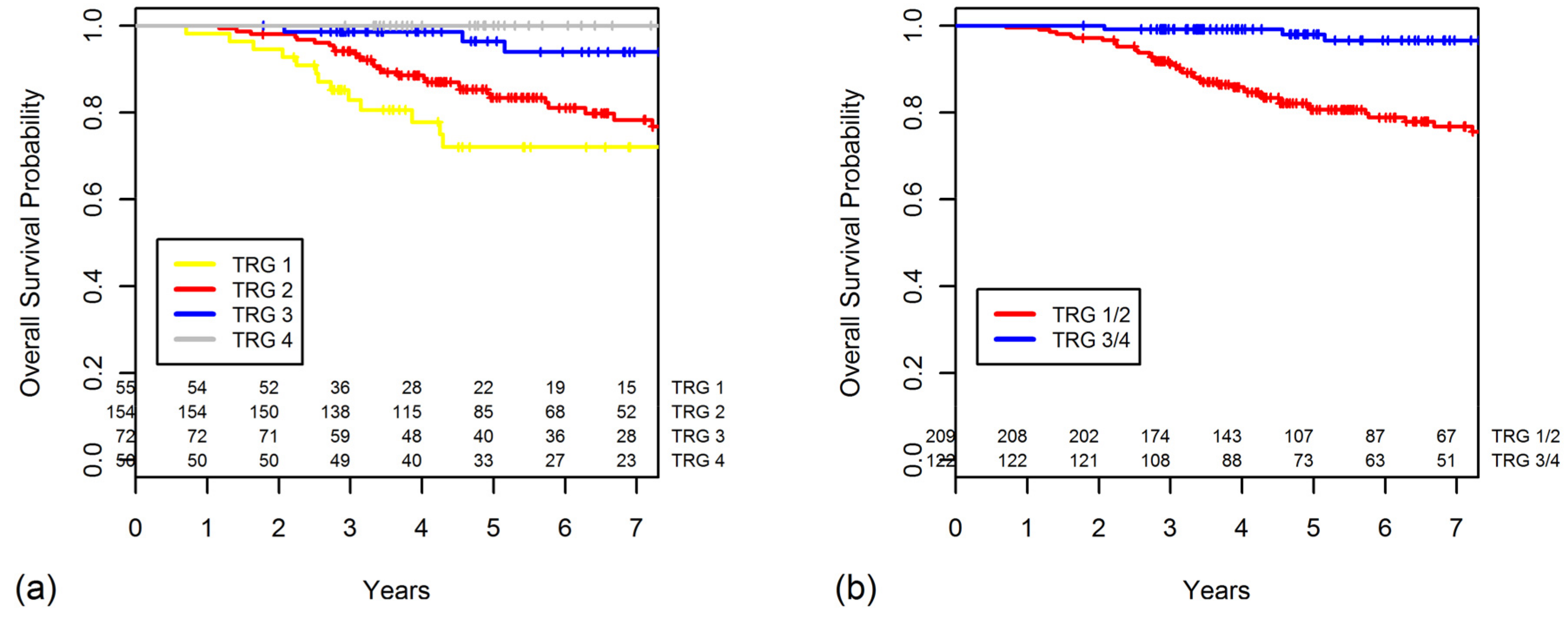
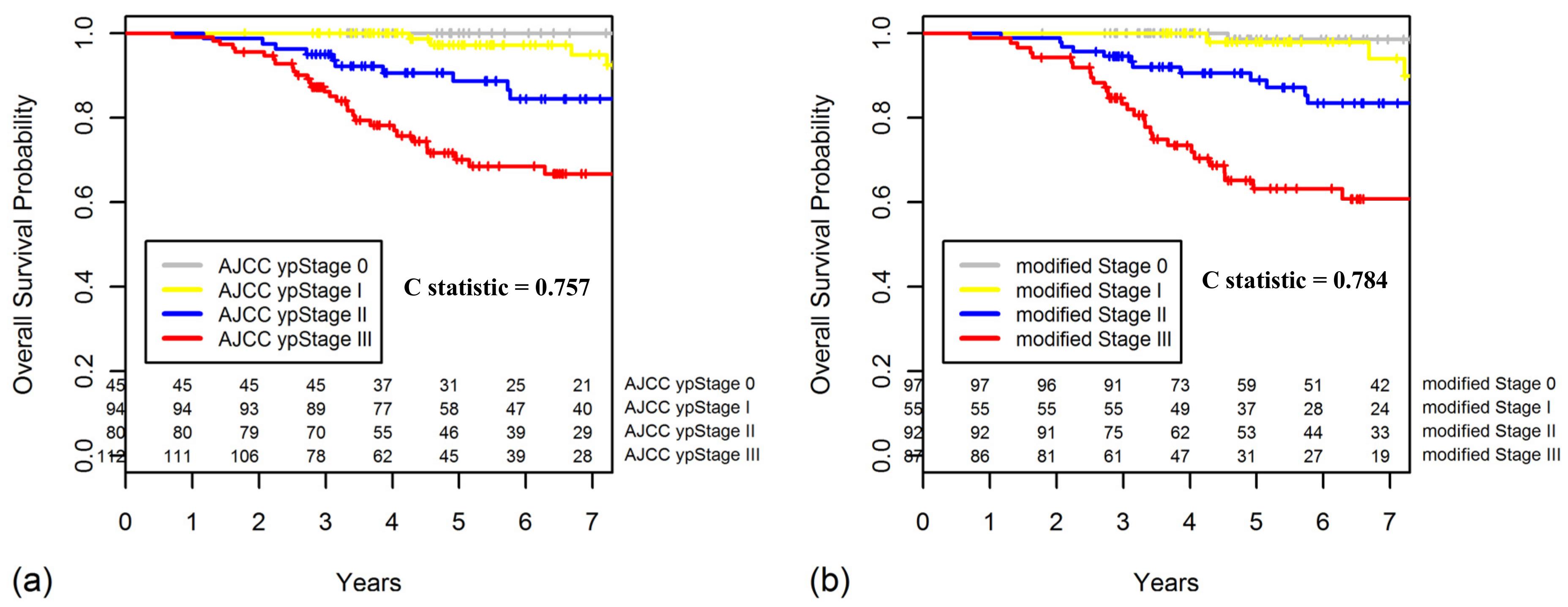
2.4. TRG as a Prognostic Factor for OS
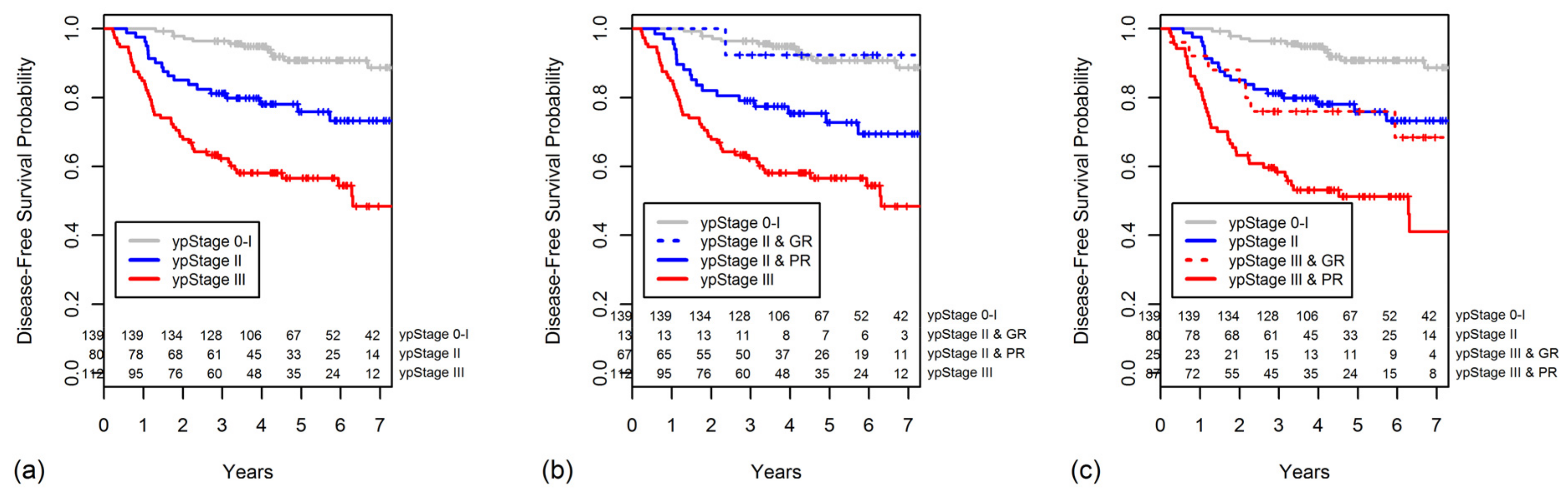
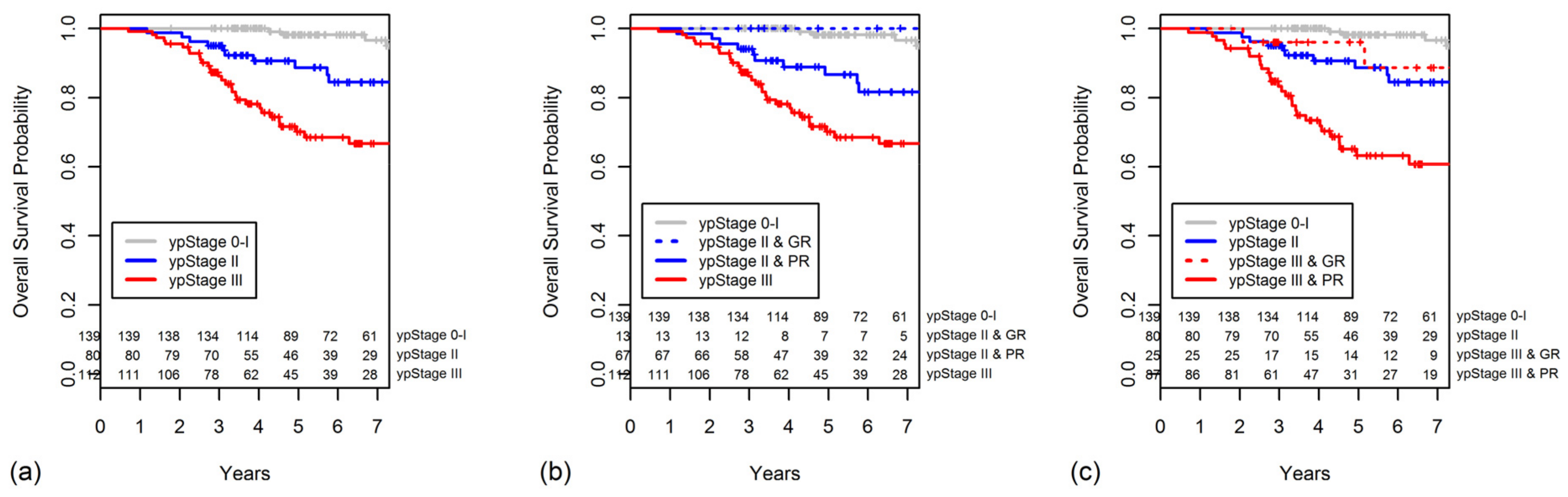
2.5. Constructing a New Modified Staging System That Combines ypStage and Grouped TRG
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Gantt, G.A.; Chen, Y.; Dejulius, K.; Mace, A.G.; Barnholtz-Sloan, J.; Kalady, M.F. Gene expression profile is associated with chemoradiation resistance in rectal cancer. Colorectal Dis. 2014, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, E.; Del Bianco, P.; Bertorelle, R.; Boso, C.; Perin, A.; Spiro, G.; Bergamo, F.; Belluco, C.; Buonadonna, A.; Palazzari, E.; et al. The predictive and prognostic potential of plasma telomerase reverse transcriptase (tert) rna in rectal cancer patients. Br. J. Cancer 2018, 118, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Pucciarelli, S.; Bertorelle, R.; De Rossi, A.; Nitti, D. Predictive factors of the response of rectal cancer to neoadjuvant radiochemotherapy. Cancers 2011, 3, 2176–2194. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Revoltar, M.; Lim, S.H.; Tut, T.G.; Ng, W.; Lee, M.; de Souza, P.; et al. Early postoperative low expression of rad50 in rectal cancer patients associates with disease-free survival. Cancers 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chang, H.J.; Kim, D.Y.; Park, J.W.; Baek, J.Y.; Kim, S.Y.; Park, S.C.; Oh, J.H.; Yu, A.; Nam, B.H. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res. Treat. 2016, 48, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the cao/aro/aio-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.M.; Valentini, V.; Minsky, B.D.; Padula, G.D.; Venkatraman, E.S.; Balducci, M.; Micciche, F.; Ricci, R.; Morganti, A.G.; Gambacorta, M.A.; et al. The relationship of pathologic tumor regression grade (Trg) and outcomes after preoperative therapy in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Kim, J.H. Controversial issues in radiotherapy for rectal cancer: A systematic review. Radiat. Oncol. J. 2017, 35, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Yu, C.S.; Hong, Y.S.; Kim, T.W.; Park, J.H.; Kim, J.H.; Kim, J.C. Failure patterns correlate with the tumor response after preoperative chemoradiotherapy for locally advanced rectal cancer. J. Surg. Oncol. 2012, 106, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.; Silva, C.; Rocha, A.; Matos, E.; Nogueira, C.; Lopes, C. Prognostic value of mandard and dworak tumor regression grading in rectal cancer: Study of a single tertiary center. ISRN Surg. 2014, 2014, 310542. [Google Scholar] [CrossRef] [PubMed]
- Swellengrebel, H.A.; Bosch, S.L.; Cats, A.; Vincent, A.D.; Dewit, L.G.; Verwaal, V.J.; Nagtegaal, I.D.; Marijnen, C.A. Tumour regression grading after chemoradiotherapy for locally advanced rectal cancer: A near pathologic complete response does not translate into good clinical outcome. Radiother. Oncol. 2014, 112, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gavioli, M.; Luppi, G.; Losi, L.; Bertolini, F.; Santantonio, M.; Falchi, A.M.; D’Amico, R.; Conte, P.F.; Natalini, G. Incidence and clinical impact of sterilized disease and minimal residual disease after preoperative radiochemotherapy for rectal cancer. Dis. Colon Rectum 2005, 48, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Mace, A.G.; Pai, R.K.; Stocchi, L.; Kalady, M.F. American joint committee on cancer and college of american pathologists regression grade: A new prognostic factor in rectal cancer. Dis. Colon Rectum 2015, 58, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Strobel, P.; Fietkau, R.; Ghadimi, M.; Liersch, T.; Grabenbauer, G.G.; Hartmann, A.; Kaufmann, M.; Sauer, R.; Graeven, U.; et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.; Warren, B.F.; Mortensen, N.J.; Ekanyaka, N.; Kulacoglu, H.; Jones, A.C.; George, B.D.; Kettlewell, M.G. Quantification of histologic regression of rectal cancer after irradiation: A proposal for a modified staging system. Dis. Colon Rectum 2002, 45, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Hsieh, C.C.; Chuang, J.P. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: A meta-analysis. Dis. Colon Rectum 2013, 56, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Guerra, G.R.; Warrier, S.K.; Lynch, A.C.; Michael, M.; Ngan, S.Y.; Phillips, W.; Ramsay, G.; Heriot, A.G. Prognostic value of tumour regression grade in locally advanced rectal cancer: A systematic review and meta-analysis. Colorectal Dis. 2018, 20, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sadahiro, S.; Tanaka, A.; Okada, K.; Saito, G.; Miyakita, H.; Akiba, T.; Yamamuro, H. A modified classification of prognostic factors based on pathological stage and tumor regression grade in patients with rectal cancer who receive preoperative chemoradiotherapy. Oncology 2017, 93, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Chang, G.J.; Hu, C.Y.; Taggart, M.; Rashid, A.; Park, I.J.; You, Y.N.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo resection after neoadjuvant chemoradiotherapy. Cancer 2013, 119, 4231–4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagkounis, G.; Thai, L.; Mace, A.G.; Wiland, H.; Pai, R.K.; Steele, S.R.; Church, J.M.; Kalady, M.F. Prognostic implications of pathological response to neoadjuvant chemoradiation in pathologic stage iii rectal cancer. Ann. Surg. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Trakarnsanga, A.; Gonen, M.; Shia, J.; Nash, G.M.; Temple, L.K.; Guillem, J.G.; Paty, P.B.; Goodman, K.A.; Wu, A.; Gollub, M.; et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Becker, K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018, 472, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Rodel, C.; Martus, P.; Papadoupolos, T.; Fuzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; Gill, P.; Govender, D.; Bateman, A.; Chang, H.J.; Deshpande, V.; Driman, D.; Gomez, M.; Greywoode, G.; Jaynes, E.; et al. International study group on rectal cancer regression grading: Interobserver variability with commonly used regression grading systems. Hum. Pathol. 2012, 43, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Van der Paardt, M.P.; Zagers, M.B.; Beets-Tan, R.G.; Stoker, J.; Bipat, S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic mr imaging: A systematic review and meta-analysis. Radiology 2013, 269, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.B.; Taylor, F.; Blomqvist, L.; George, C.; Evans, H.; Tekkis, P.; Quirke, P.; Sebag-Montefiore, D.; Moran, B.; Heald, R.; et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: Mercury experience. J. Clin. Oncol. 2011, 29, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Counsell, N.; Quirke, P.; Mortensen, N.; Maraveyas, A.; Meadows, H.M.; Ledermann, J.; Sebag-Montefiore, D. Chronicle: Results of a randomised phase iii trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (xelox) versus control. Ann. Oncol. 2014, 25, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Breugom, A.J.; van Gijn, W.; Muller, E.W.; Berglund, A.; van den Broek, C.B.; Fokstuen, T.; Gelderblom, H.; Kapiteijn, E.; Leer, J.W.; Marijnen, C.A.; et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A dutch colorectal cancer group (dccg) randomized phase iii trial. Ann. Oncol. 2015, 26, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Sainato, A.; Cernusco Luna Nunzia, V.; Valentini, V.; De Paoli, A.; Maurizi, E.R.; Lupattelli, M.; Aristei, C.; Vidali, C.; Conti, M.; Galardi, A.; et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (larc): Long term results of a randomized trial (i-cnr-rt). Radiother. Oncol. 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Stojanovic-Rundic, S.; Bensadoun, R.J.; Bardet, E.; Beny, A.; Ollier, J.C.; Bolla, M.; et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the eortc 22921 randomised study. Lancet Oncol. 2014, 15, 184–190. [Google Scholar] [CrossRef]
- Collette, L.; Bosset, J.F.; den Dulk, M.; Nguyen, F.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Pierart, M.; Calais, G.; European Organisation for, R.; et al. Patients with curative resection of ct3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the european organisation for research and treatment of cancer radiation oncology group. J. Clin. Oncol. 2007, 25, 4379–4386. [Google Scholar] [PubMed]
- Hong, Y.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, S.J.; Park, Y.S.; Park, J.O.; Kim, S.Y.; Kim, T.Y.; Kim, J.H.; et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (adore): An open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014, 15, 1245–1253. [Google Scholar] [CrossRef]
- Jang, N.Y.; Kang, S.B.; Kim, D.W.; Kim, J.H.; Lee, K.W.; Kim, I.A.; Kim, J.S. The role of carcinoembryonic antigen after neoadjuvant chemoradiotherapy in patients with rectal cancer. Dis. Colon Rectum 2011, 54, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.B.; Park, J.W.; Jeong, S.Y.; Nam, B.H.; Choi, H.S.; Kim, D.W.; Lim, S.B.; Lee, T.G.; Kim, D.Y.; Kim, J.S.; et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (corean trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010, 11, 637–645. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, K.H.; Lee, H.J.; Ha, K.; Lim, C.; Chin, H.J.; Yun, J.; Cho, E.Y.; Chung, E.; Baek, R.M.; et al. Seoul national university bundang hospital’s electronic system for total care. Healthc. Inform. Res. 2012, 18, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; Bhoday, J.; Battersby, N.J.; Chand, M.; West, N.P.; Abulafi, A.M.; Tekkis, P.P.; Brown, G. Defining response to radiotherapy in rectal cancer using magnetic resonance imaging and histopathological scales. World J. Gastroenterol. 2016, 22, 8414–8434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elezkurtaj, S.; Moser, L.; Budczies, J.; Muller, A.J.; Blaker, H.; Buhr, H.J.; Dietel, M.; Kruschewski, M. Histopathological regression grading matches excellently with local and regional spread after neoadjuvant therapy of rectal cancer. Pathol. Res. Pract. 2013, 209, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Durdey, P.; Dixon, M.F.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 2, 996–999. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, D.Y.; Park, J.W.; Oh, J.H.; Chang, H.J.; Kim, S.Y.; Kim, T.H.; Park, H.C.; Choi, D.H.; Chun, H.K.; et al. Can the new american joint committee on cancer staging system predict survival in rectal cancer patients treated with curative surgery following preoperative chemoradiotherapy? Cancer 2012, 118, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Quah, H.M.; Chou, J.F.; Gonen, M.; Shia, J.; Schrag, D.; Saltz, L.B.; Goodman, K.A.; Minsky, B.D.; Wong, W.D.; Weiser, M.R. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008, 113, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, L.; Wang, C.; Huang, R.; Peng, H.; Zhang, T.; Dong, J.; Xiao, W.; Zeng, Z.; Liu, M.; et al. Prognostic significance of clinical and pathological stages on locally advanced rectal carcinoma after neoadjuvant chemoradiotherapy. Radiat. Oncol. 2015, 10, 124. [Google Scholar] [CrossRef] [PubMed]
| Variable | GR (n = 122) | PR (n = 209) | p Value |
|---|---|---|---|
| Age at diagnosis | 0.404 | ||
| ≤61 years | 63 (51.6%) | 98 (46.9%) | |
| >61 years | 59 (48.4%) | 111 (53.1%) | |
| Sex | 0.553 | ||
| Male | 82 (67.2%) | 147 (70.3%) | |
| Female | 40 (32.8%) | 62 (29.7%) | |
| Clinical T classification | 0.018 | ||
| cT2 | 12 (9.8%) | 8 (3.8%) | |
| cT3 | 103 (84.4%) | 175 (83.7%) | |
| cT4 | 7 (5.7%) | 26 (12.4%) | |
| Clinical N classification | 0.256 | ||
| cN0 | 22 (18.0%) | 28 (13.4%) | |
| cN+ | 100 (82.0%) | 181 (86.6%) | |
| Distance from anal verge | 0.747 | ||
| ≤5 cm | 74 (60.7%) | 123 (58.9%) | |
| >5 cm | 48 (39.3%) | 86 (41.1%) | |
| Type of surgery | 0.012 | ||
| Sphincter-preserving surgery | 115 (94.3%) | 178 (85.2%) | |
| Abdominoperineal resection | 7 (5.7%) | 31 (14.8%) | |
| Interval completion of CRT to surgery | 0.029 | ||
| <42 days | 7 (5.7%) | 28 (13.4%) | |
| ≥42 days | 115 (94.3%) | 181 (86.6%) | |
| Adjuvant chemotherapy | 0.396 | ||
| No | 13 (10.7%) | 29 (13.9%) | |
| Yes | 109 (89.3%) | 180 (86.1%) | |
| Regimen of adjuvant chemotherapy | 0.027 | ||
| Single agent | 88 (80.7%) | 124 (68.9%) | |
| Combination | 21 (19.3%) | 56 (31.1%) | |
| Lymphatic invasion | 0.007 | ||
| No | 115 (95.8%) | 181 (86.6%) | |
| Yes | 5 (4.2%) | 28 (13.4%) | |
| Venous invasion | 0.001 | ||
| No | 118 (98.3%) | 184 (88.0%) | |
| Yes | 2 (1.7%) | 25 (12.0%) | |
| Perineural invasion | <0.001 | ||
| No | 114 (95.0%) | 148 (70.8%) | |
| Yes | 6 (5.0%) | 61 (29.2%) | |
| ypT classification | <0.001 | ||
| ypT0 | 50 (41.0%) | 0 (0%) | |
| ypT1 | 14 (11.5%) | 8 (3.8%) | |
| ypT2 | 31 (25.4%) | 60 (28.7%) | |
| ypT3 | 26 (21.3%) | 138 (66.0%) | |
| ypT4 | 1 (0.8%) | 3 (1.4%) | |
| ypN classification | <0.001 | ||
| ypN0 | 97 (79.5%) | 122 (58.4%) | |
| ypN1 | 18 (14.8%) | 69 (33.0%) | |
| ypN2 | 7 (5.7%) | 18 (8.6%) | |
| ypTN classification | <0.001 | ||
| ypT0N0 | 45 (36.9%) | 0 (0%) | |
| ypT1–2N0 | 39 (32.0%) | 55 (26.3%) | |
| ypT3–4N0 | 13 (10.7%) | 67 (32.1%) | |
| ypT0N+ | 5 (4.1%) | 0 (0%) | |
| ypT1–2N1 | 6 (4.9%) | 13 (6.2%) | |
| ypT3–4N1 | 10 (8.2%) | 56 (26.8%) | |
| ypT1–4N2 | 4 (3.3%) | 18 (8.6%) | |
| Number of harvested lymph nodes a | |||
| All patients | 25 (20–31) | 24 (18–34) | 0.813 |
| ypStage 0 | 25 (20–30) | – | – |
| ypStage I | 25 (21–37) | 21 (16–31) | 0.020 |
| ypStage II | 24 (20–30) | 26 (19–33) | 0.092 |
| ypStage III | 28 (19–34) | 25 (19–36) | 0.942 |
| ypStage | <0.001 | ||
| 0 | 45 (36.9%) | 0 (0%) | |
| I | 39 (32.0%) | 55 (26.3%) | |
| II | 13 (10.7%) | 67 (32.1%) | |
| III | 25 (20.5%) | 87 (41.6%) | |
| Circumferential resection margin | 0.013 | ||
| >1 mm | 119 (97.5%) | 189 (90.4%) | |
| ≤1 mm | 3 (2.5%) | 20 (9.6%) |
| Variables | No. of Patients | 5-Year DFS (%) | p Value | 5-Year OS (%) | p Value |
|---|---|---|---|---|---|
| Age at diagnosis | 0.084 | 0.012 | |||
| ≤61 years | 161 | 79.7 | 91.5 | ||
| >61 years | 170 | 72.0 | 82.6 | ||
| Sex | 0.481 | 0.335 | |||
| Male | 229 | 75.5 | 86.3 | ||
| Female | 102 | 75.8 | 88.0 | ||
| Clinical T classification | 0.040 | 0.115 | |||
| cT2 | 20 | 88.2 | 100 | ||
| cT3 | 278 | 75.5 | 85.6 | ||
| cT4 | 33 | 69.7 | 90.7 | ||
| Clinical N classification | 0.016 | 0.054 | |||
| cN0 | 50 | 90.4 | 95.1 | ||
| cN+ | 281 | 73.0 | 85.4 | ||
| Distance from anal verge | 0.485 | 0.909 | |||
| ≤5 cm | 197 | 73.8 | 87.1 | ||
| >5 cm | 134 | 78.4 | 86.5 | ||
| Type of surgery | 0.658 | 0.245 | |||
| Sphincter-preserving surgery | 293 | 75.8 | 87.2 | ||
| Abdominoperineal resection | 38 | 75.0 | 84.1 | ||
| Interval completion of CRT to surgery | 0.157 | 0.329 | |||
| <42 days | 35 | 68.3 | 82.5 | ||
| ≥42 days | 296 | 76.5 | 87.4 | ||
| Adjuvant chemotherapy | 0.549 | 0.765 | |||
| No | 42 | 71.2 | 86.6 | ||
| Yes | 289 | 76.3 | 86.9 | ||
| Lymphatic invasion | 0.001 | 0.001 | |||
| No | 296 | 77.4 | 88.8 | ||
| Yes | 33 | 57.6 | 68.6 | ||
| Venous invasion | <0.001 | 0.002 | |||
| No | 302 | 77.6 | 88.6 | ||
| Yes | 27 | 51.6 | 63.9 | ||
| Perineural invasion | <0.001 | <0.001 | |||
| No | 262 | 82.3 | 92.2 | ||
| Yes | 67 | 48.3 | 63.3 | ||
| ypT classification | <0.001 | <0.001 | |||
| ypT0 | 50 | 96.0 | 100 | ||
| ypT1 | 22 | 94.1 | 100 | ||
| ypT2 | 91 | 81.2 | 93.6 | ||
| ypT3 | 164 | 64.4 | 77.4 | ||
| ypT4 | 4 | 50.0 | 75.0 | ||
| ypN classification | <0.001 | <0.001 | |||
| ypN0 | 219 | 85.3 | 94.7 | ||
| ypN1 | 87 | 59.2 | 73.7 | ||
| ypN2 | 25 | 47.7 | 58.5 | ||
| ypStage | <0.001 | <0.001 | |||
| 0 | 45 | 97.8 | 100 | ||
| I | 94 | 87.0 | 97.2 | ||
| II | 80 | 75.9 | 88.7 | ||
| III | 112 | 56.6 | 70.1 | ||
| Circumferential resection margin | <0.001 | <0.001 | |||
| >1 mm | 308 | 77.8 | 88.4 | ||
| ≤1 mm | 23 | 47.1 | 65.7 | ||
| Tumor regression grade | <0.001 | <0.001 | |||
| 4 | 50 | 96.0 | 100 | ||
| 3 | 72 | 87.8 | 96.4 | ||
| 2 | 154 | 69.2 | 83.4 | ||
| 1 | 55 | 59.1 | 72.0 | ||
| 0 | 0 | – | – | ||
| Grouped tumor regression grade | <0.001 | <0.001 | |||
| 3/4 | 122 | 91.3 | 98.0 | ||
| 1/2 | 209 | 66.6 | 80.6 |
| Variables | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Grouped TRG | ||||
| 1/2 | Ref | Ref | ||
| 3/4 | 0.35 (0.19–0.66) | 0.001 | 0.29 (0.10–0.84) | 0.023 |
| ypN classification | ||||
| ypN0 | Ref | Ref | ||
| ypN1 | 2.33 (1.42–3.83) | 0.001 | 1.77 (0.88–3.56) | 0.109 |
| ypN2 | 3.71 (1.93–7.14) | <0.001 | 3.21 (1.40–7.35) | 0.006 |
| Perineural invasion | ||||
| No | Ref | Ref | ||
| Yes | 2.60 (1.63–4.14) | <0.001 | 2.26 (1.21–4.23) | 0.011 |
| Age | — | — | 1.04 (1.01–1.07) | 0.008 |
| ypT classification | ||||
| ypT0–2 | Ref | |||
| ypT3–4 | — | — | 2.08 (0.89–4.85) | 0.091 |
| CRM | ||||
| >1 mm | Ref | |||
| ≤1 mm | — | — | 2.08 (0.95–4.56) | 0.067 |
| modified Stage 0 | ypStage 0–I & TRG 3/4, ypStage II & TRG 3/4 |
| modified Stage I | ypStage 0–I & TRG 1/2 |
| modified Stage II | ypStage II & TRG 1/2, ypStage III & TRG 3/4 |
| modified Stage III | ypStage III & TRG 1/2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Chung, J.-H.; Kang, S.-B.; Kim, D.-W.; Oh, H.-K.; Lee, H.S.; Kim, J.W.; Lee, K.-W.; Kim, J.H.; Kim, J.-S. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers 2018, 10, 319. https://doi.org/10.3390/cancers10090319
Song C, Chung J-H, Kang S-B, Kim D-W, Oh H-K, Lee HS, Kim JW, Lee K-W, Kim JH, Kim J-S. Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System. Cancers. 2018; 10(9):319. https://doi.org/10.3390/cancers10090319
Chicago/Turabian StyleSong, Changhoon, Joo-Hyun Chung, Sung-Bum Kang, Duck-Woo Kim, Heung-Kwon Oh, Hye Seung Lee, Jin Won Kim, Keun-Wook Lee, Jee Hyun Kim, and Jae-Sung Kim. 2018. "Impact of Tumor Regression Grade as a Major Prognostic Factor in Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy: A Proposal for a Modified Staging System" Cancers 10, no. 9: 319. https://doi.org/10.3390/cancers10090319





