Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients
Abstract
:1. Introduction
2. Results
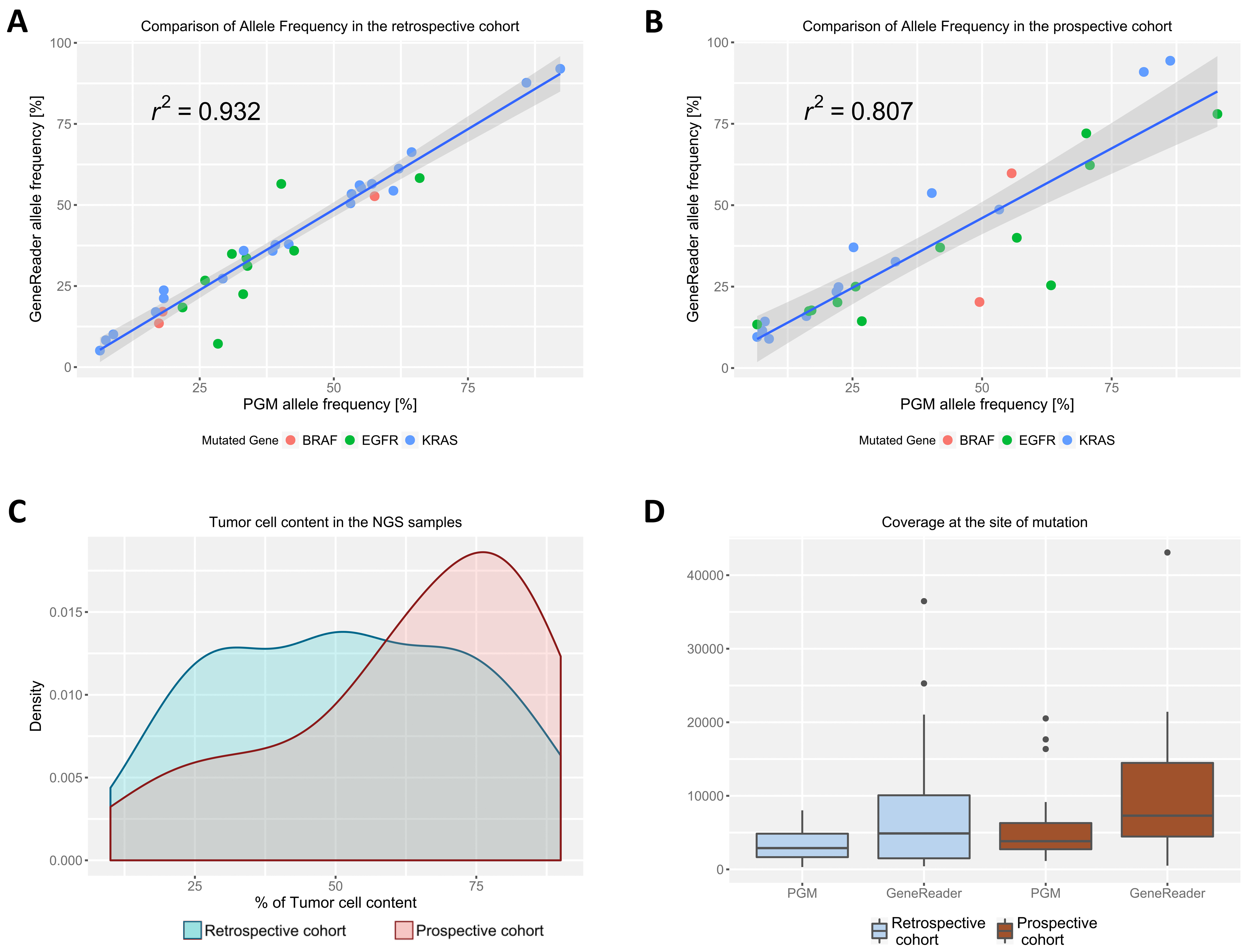
2.1. GeneReader Reveals High Concordance with Ion PGM
2.2. Allele Frequency between the Sequencing Systems Is Highly Correlated
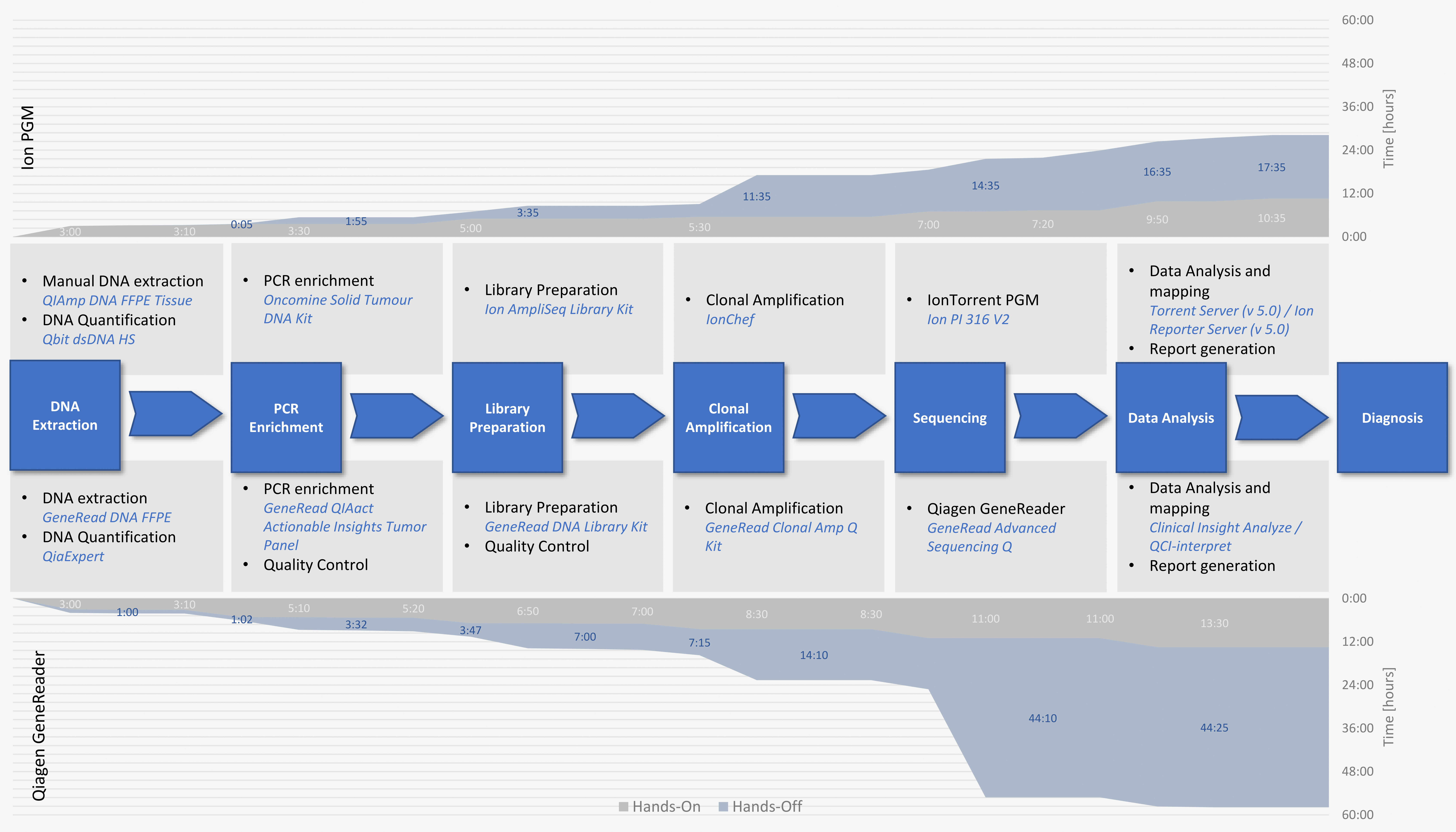
2.3. GeneReader and Ion PGM Show Comparable Hands-on Time but Different Total Run Times
3. Discussion
4. Materials and Methods
4.1. Sample and DNA Isolation
4.2. Ion PGM Assay and Sequencing
4.3. GeneReader Assay and Sequencing
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.i.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib Versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ngoc, T.; Bouchaab, H.; Adjei, A.A.; Peters, S. BRAF Alterations as Therapeutic Targets in Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. Metastatic Non-Small-Cell Lung Cancer: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2016, 27, v1–v27. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.E.; Zhang, L.; Wang, J.; Smith, G.H.; Newman, S.; Schneider, T.M.; Pillai, R.N.; Kudchadkar, R.R.; Owonikoko, T.K.; Ramalingam, S.S.; et al. Clinical Validation and Implementation of a Targeted Next-Generation Sequencing Assay to Detect Somatic Variants in Non-Small Cell Lung, Melanoma, and Gastrointestinal Malignancies. J. Mol. Diagn. 2016, 18, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.A.; Grand, D.; Rouquette, I.; Costes, V.; Icher, S.; Selves, J.; Larrieux, M.; Barbe, A.; Brousset, P.; Solassol, J. High-throughput detection of clinically targetable alterations using next-generation sequencing. Oncotarget 2017, 8, 40345–40358. [Google Scholar] [CrossRef] [PubMed]
- Sikkema-Raddatz, B.; Johansson, L.F.; De Boer, E.N.; Almomani, R.; Boven, L.G.; van den Berg, M.P.; Van Spaendonck-Zwarts, K.Y.; van Tintelen, J.P.; Sijmons, R.H. Targeted Next-Generation Sequencing can Replace Sanger Sequencing in Clinical Diagnostics. Hum. Mutat. 2013, 34, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, C.E.; Al-Kateb, H.; Bredemeyer, A.J.; Duncavage, E.J.; Spencer, D.H.; Abel, H.J.; Lockwood, C.M.; Hagemann, I.S.; O’guin, S.M.; Burcea, L.C.; et al. Validation of a next-generation sequencing assay for clinical molecular oncology. J. Mol. Diagn. 2014, 16, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Mosier, S.L.; Thiess, M.; Beierl, K.F.; Debeljak, M.; Tseng, L.-H.; Chen, G.; Yegnasubramanian, S.; Ho, H.; Cope, L.; et al. Clinical Validation of KRAS, BRAF, and EGFR Mutation Detection Using Next-Generation Sequencing. Am. J. Clin. Pathol. 2014, 141, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Patel, K.P.; Routbort, M.J.; Reddy, N.G.; Barkoh, B.A.; Handal, B.; Kanagal-Shamanna, R.; Greaves, W.O.; Medeiros, L.J.; Aldape, K.D.; et al. Clinical Validation of a Next-Generation Sequencing Screen for Mutational Hotspots in 46 Cancer-Related Genes. J. Mol. Diagn. 2013, 15, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, J.W.J.; Van Blokland, W.T.M.; Moons, M.J.; Radersma, R.D.; Radersma-van Loon, J.H.; De Voijs, C.M.A.; Rappel, S.B.; Koudijs, M.J.; Besselink, N.J.M.; Willems, S.M.; et al. Comparison of Next-Generation Sequencing and Mutation-Specific Platforms in Clinical Practice. Am. J. Clin. Pathol. 2015, 143, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Cofrac-Comité Français D’accréditation. Available online: http://www.cofrac.fr/fr/organismes/fiche.php?entite_id=82017619 (accessed on 18 December 2017).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’amico, T.A.; Decamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef]
- Séquençage de Nouvelle Génération d’un Panel de Gènes Pour L’analyse en Génétique Somatique/Validation de la Méthode. 2016. Available online: http://www.e-cancer.fr/content/download/148150/1859133/file/validation%20de%20m%c3%a9thode%20ngs_fr%20final%20compil%c3%a9.pdf (accessed on 19 March 2018).
- Darwanto, A.; Hein, A.-M.; Strauss, S.; Kong, Y.; Sheridan, A.; Richards, D.; Lader, E.; Ngowe, M.; Pelletier, T.; Adams, D.; et al. Use of the QIAGEN GeneReader NGS System for Detection of KRAS Mutations, Validated by the QIAGEN Therascreen PCR Kit and Alternative NGS Platform. BMC Cancer 2017, 17, 358. [Google Scholar] [CrossRef] [PubMed]
- Koitzsch, U.; Heydt, C.; Attig, H.; Immerschitt, I.; Merkelbach-Bruse, S.; Fammartino, A.; Büttner, R.H.; Kong, Y.; Odenthal, M. Use of the Genereader NGS System in a Clinical Pathology Laboratory: A Comparative Study. J. Clin. Pathol. 2017, 70, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, W.-Y.; Kim, N.K.D.; Jang, S.J.; Chun, S.-M.; Sung, C.-O.; Choi, J.; Ko, Y.-H.; Choi, Y.-L.; Shim, H.S.; et al. Good Laboratory Standards for Clinical Next-Generation Sequencing Cancer Panel Tests. J. Pathol. Transl. Med. 2017, 51, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Endrullat, C.; Glökler, J.; Franke, P.; Frohme, M. Standardization and Quality Management in Next-Generation Sequencing. Appl. Transl. Genom. 2016, 10, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Dearden, S.; Mccormack, R. EGFR Mutation Incidence in Non-Small-Cell Lung Cancer of Adenocarcinoma Histology: A Systematic Review and Global Map by Ethnicity (Mutmapii). Am. J. Cancer Res. 2015, 5, 2892–2911. [Google Scholar] [PubMed]
- De Pas, T.; Toffalorio, F.; Manzotti, M.; Fumagalli, C.; Spitaleri, G.; Catania, C.; Delmonte, A.; Giovannini, M.; Spaggiari, L.; De Braud, F.; et al. Activity of Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Patients with Non-Small Cell Lung Cancer Harboring Rare Epidermal Growth Factor Receptor Mutations. J. Thorac. Oncol. 2011, 6, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Heigener, D.F.; Schumann, C.; Sebastian, M.; Sadjadian, P.; Stehle, I.; Märten, A.; Lüers, A.; Griesinger, F.; Scheffler, M. Afatinib Compassionate Use Consortium (ACUC). Afatinib in Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations Pretreated with Reversible EGFR Inhibitors. Oncologist 2015, 20, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, I.; Aziz, N.; Farkas, D.H.; Furtado, M.; Gonzalez, A.F.; Greiner, T.C.; Grody, W.W.; Hambuch, T.; Kalman, L.; Kant, J.A.; et al. Opportunities and Challenges Associated with Clinical Diagnostic Genome Sequencing: A Report of the Association for Molecular Pathology. J. Mol. Diagn. 2012, 14, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Pongor, L.; Harami-Papp, H.; Méhes, E.; Czirók, A.; Győrffy, B. Cell Dispersal Influences Tumor Heterogeneity and Introduces a Bias in NGS Data Interpretation. Sci. Rep. 2017, 7, 7358. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-C.; Zhang, Q.; Huang, Y.-H.; Liu, Y.-F.; Chen, H.-L. Comparison of Two Methods to Extract DNA From Formalin-Fixed, Paraffin-Embedded Tissues and Their Impact on EGFR Mutation Detection in Non-Small Cell Lung Carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.I.; Hofman, V.; Bonnetaud, C.; Havet, K.; Lespinet-Fabre, V.; Coëlle, C.; Gavric-Tanga, V.; Vénissac, N.; Mouroux, J.; Hofman, P. Usefulness of Tissue Microarrays for Assessment of Protein Expression, Gene Copy Number and Mutational Status of EGFR in Lung Adenocarcinoma. Virchows Arch. 2010, 457, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.T.; Heeke, S.; Hofman, V.; Lespinet, V.; Ribeyre, C.; Bordone, O.; Poudenx, M.; Otto, J.; Garnier, G.; Castelnau, O.; et al. NGS Analysis on Tumor Tissue and cfDNA for Genotype-Directed Therapy in Metastatic NSCLC Patients. Between Hope and Hype? Expert Rev. Anticancer Ther. 2017, 17, 681–685. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Dortmund, Germany, 2008; p. 409. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; p. 213. [Google Scholar] [CrossRef]
| Ion Oncomine Panel on PGM | QIAGEN Actionable Insight Tumor Panel on GeneReader | |
|---|---|---|
| Panel Size | 22 genes; 11 kb | 12 genes; 16.7 kb |
| Gene list # | EGFR, ALK, ERBB2, ERBB4, FGFR1, FGFR2, FGFR3, MET, DDR2, KRAS, PIK3CA, BRAF, AKT1, PTEN, NRAS, MAP2K1, STK11, NOTCH1, CTNNB1, SMAD4, FBXW7, TP53 | KRAS, NRAS, KIT, BRAF, PDGFRA, ALK, EGFR, ERBB2, PIK3CA, ERBB3, ESR1, RAF1 |
| Amplicons | 92 | 330 |
| Variant allele fraction detection limit | 5% | 5% |
| DNA amount | 10 ng | 40 ng |
| Minimal base coverage depth | >300X ǂ | Not specified by the manufacturer |
| Label | CE IVD | RUO |
| Mutation Status | Retrospective Cohort [N = 60] | Prospective Cohort [N = 30] |
|---|---|---|
| No detected mutations | 25 (42%) | 5 (17%) |
| Mutations detected in | 33 (55%) | 25 (83%) |
| EGFR | 9 (15%) | 8 (27%) |
| KRAS | 20 (33%) | 13 (43%) |
| BRAF | 3 (5%) | 2 (7%) |
| Double Mutations | ||
| EGFR del19 & KRAS p.G12D | 1 (2%) | na |
| EGFR p.V774M & p.L861Q | na | 1 (3%) |
| EGFR p.L858R & p.G719C | na | 1 (3%) |
| Failed analysis | 2 (3%) | na |
| Retrospective Cohort | Prospective Cohort | Total | |
|---|---|---|---|
| N | 60 (67%) | 30 (33%) | 90 (100%) |
| Tumor Tissue | |||
| Frozen | 12 (20%) | na | 12 (13%) |
| FFPE | 48 (80%) | 30 (100%) | 78 (87 %) |
| Age | |||
| Median (range) | 67 (50–90) | 69 (47–86) | 68 (47–90) |
| Sex | |||
| Female | 24 (40%) | 13 (43%) | 37 (41%) |
| Male | 36 (60%) | 17 (57%) | 53 (59%) |
| Smoking | |||
| Current smoker | 24 (40%) | 5 (17%) | 29 (32%) |
| Former smoker | 18 (30%) | 8 (27%) | 26 (29%) |
| Non-smoker | 5 (8%) | 6 (20%) | 11 (12%) |
| Unknown | 13 (22%) | 11 (37%) | 24 (27%) |
| Tumor-cell content Median (range) [%] | 50 (10–90) | 70 (10–90) | 60 (10–90) |
| TNM stage | |||
| II | 11 (18%) | 7 (23%) | 18 (20%) |
| III | 11 (18%) | 3 (10%) | 14 (16%) |
| IV | 38 (63%) | 20 (67%) | 58 (64%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heeke, S.; Hofman, V.; Long-Mira, E.; Lespinet, V.; Lalvée, S.; Bordone, O.; Ribeyre, C.; Tanga, V.; Benzaquen, J.; Leroy, S.; et al. Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients. Cancers 2018, 10, 88. https://doi.org/10.3390/cancers10040088
Heeke S, Hofman V, Long-Mira E, Lespinet V, Lalvée S, Bordone O, Ribeyre C, Tanga V, Benzaquen J, Leroy S, et al. Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients. Cancers. 2018; 10(4):88. https://doi.org/10.3390/cancers10040088
Chicago/Turabian StyleHeeke, Simon, Véronique Hofman, Elodie Long-Mira, Virginie Lespinet, Salomé Lalvée, Olivier Bordone, Camille Ribeyre, Virginie Tanga, Jonathan Benzaquen, Sylvie Leroy, and et al. 2018. "Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients" Cancers 10, no. 4: 88. https://doi.org/10.3390/cancers10040088





