NK Cell-Based Glioblastoma Immunotherapy
Abstract
:1. Introduction
2. Glioblastoma
3. Mechanisms of Immunosuppression
4. The Role of NK Cells on GB
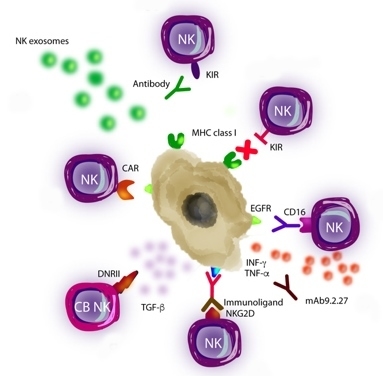
5. Therapeutic Approaches in GB with NK Cells
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood-brain barrier |
| CAR | Chimeric antigen receptor |
| CB | Cord blood |
| CSF | Cerebrospinal fluid |
| CNS | Central Nervous System |
| CTL | Cytotoxic T lymphocyte |
| CVO | Circumventricular organ |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| DISC | Death-inducing signaling complex |
| DNRII | Dominant negative TGF-β receptor II |
| FADD | Fas-associated death receptor |
| FasL | Fas-ligand |
| GB | glioblastoma |
| HDACi | Histone deacetylase inhibitor |
| HLA | Human leukocyte antigen |
| IL | Interleukin |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| ITIM | Immunoreceptor tyrosine-based inhibition motif |
| ITSM | Immunoreceptor tyrosine-based switch motif |
| ITT | Immunoreceptor tyrosine tail |
| KIR | Killer immunoglobulin-like receptor |
| LAK | Lymphokine-activated killer |
| MHC | Major histocompatibility complex |
| MICA | MHC class I polypeptide-related sequence A |
| MMP | matrix metalloproteinase |
| MTOC | Microtubule-organizing center |
| NK | Natural Killer |
| PBMC | Peripheral blood mononuclear cell |
| PDGF | Platelet-derived growth factor |
| PDGFR | Platelet-derived growth factor receptor beta |
| PGE2 | Prostaglandin E2 |
| SHP-1 | Src homology region 2 domain-containing phosphatase-1 |
| TAM | Tumor-associated macrophage |
| TCR | T-Cell receptor |
| TGF-β | Transforming Growth Factor beta |
| TMZ | Temozolomide |
| TRAIL | Tumor necrosis factor-related apoptosis-induced ligand |
| Treg | Tregulatory lymphocyte |
| VEGF | Vascular endothelial growth factor |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiology Biomarkers and Prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Kalman, B. Molecular Heterogeneity of Glioblastoma and Its Clinical Relevance. Pathol. Oncol. Res. 2014, 20, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Burrell, K.E.; Wolf, A.; Jalali, S.; Hawkins, C.; Rutka, J.T.; Zadeh, G. Glioblastoma, a Brief Review of History, Molecular Genetics, Animal Models and Novel Therapeutic Strategies. Arch. Immunol. Ther. Exp. 2013, 61, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Magaña-Maldonado, R.; Chávez-Cortez, E.G.; Olascoaga-Arellano, N.K.; López-Mejía, M.; Maldonado-Leal, F.M.; Sotelo, J.; Pineda, B. Immunological Evasion in Glioblastoma. BioMed Res. Int. 2016, 2016, 7487313. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B. Regulation of Immune Cell Entry into the Central Nervous System. In Results and Problems in Cell Differentiation; Richter, D., Tiedge, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 259–280. [Google Scholar]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef] [PubMed]
- Fooksman, D.R.; Vardhana, S.; Vasiliver-Shamis, G.; Liese, J.; Blair, D.A.; Waite, J.; Sacristán, C.; Victora, G.D.; Zanin-Zhorov, A.; Dustin, M.L. Functional Anatomy of T Cell Activation and Synapse Formation. Annu. Rev. Immunol. 2010, 28, 79–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubeník, J. Tumour MHC Class I Downregulation and Immunotherapy (Review). Oncol. Rep. 2003, 10, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Coussens, L.M. The Inflammatory Tumor Microenvironment and Its Impact on Cancer Development. Contrib. Microbiol. 2006, 13, 118–137. [Google Scholar] [PubMed]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional Communication in the Microenvirons of Glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Ahn, B.J.; Han, S.J.; Parsa, A.T. Soluble Factors Secreted by Glioblastoma Cell Lines Facilitate Recruitment, Survival, and Expansion of Regulatory T Cells: Implications for Immunotherapy. Neuro Oncol. 2012, 14, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, Y.; Diserens, A.C.; de Tribolet, N. In Vitro Prostaglandin E2 Production by Glioblastoma Cells and Its Effect on Interleukin-2 Activation of Oncolytic Lymphocytes. J. Neurooncol. 1990, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Canazza, A.; Rizzo, A.; Mossa, A.; Corsini, E.; Silvani, A.; Fariselli, L.; Salmaggi, A.; Ciusani, E. Circulating T Regulatory Cells Migration and Phenotype in Glioblastoma Patients: An in Vitro Study. J. Neurooncol. 2013, 115, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Betz, A.G. The Role of Regulatory T Cells in Alloantigen Tolerance. Immunol. Rev. 2006, 212, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Barr, J.; Kong, L.-Y.; Wang, Y.; Wu, A.; Sharma, A.K.; Gumin, J.; Henry, V.; Colman, H.; Priebe, W.; et al. Glioblastoma Cancer-Initiating Cells Inhibit T-Cell Proliferation and Effector Responses by the Signal Transducers and Activators of Transcription 3 Pathway. Mol. Cancer Ther. 2010, 9, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, D.; Balça-Silva, J.; da Graça, G.C.; Wanjiru, C.M.; Macharia, L.W.; Nascimento, C.P.; Roque, N.R.; Coelho-Aguiar, J.M.; Pereira, C.M.; Dos Santos, M.F.; et al. Microglia/Astrocytes–Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front. Cell. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, J.; Kocyk, M.; Nauman, P.; Gabrusiewicz, K.; Sielska, M.; Przanowski, P.; Maleszewska, M.; Rajan, W.D.; Pszczolkowska, D.; Tykocki, T.; et al. Down-Regulation of IKKβ expression in Glioma-Infiltrating Microglia/Macrophages Is Associated with Defective Inflammatory/Immune Gene Responses in Glioblastoma. Oncotarget 2015, 6, 33077–33090. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Parvani, J.G.; Schiemann, W.P. The Relevance of the TGF-β Paradox to EMT-MET Programs. Cancer Lett. 2013, 341, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xia, T.; Wang, D.; Huang, B.; Zhao, P.; Wang, J.; Qu, X.; Li, X. Human Astrocytes Secrete IL-6 to Promote Glioma Migration and Invasion through Upregulation of Cytomembrane MMP14. Oncotarget 2016, 7, 62425–62438. [Google Scholar] [CrossRef] [PubMed]
- Held-Feindt, J.; Hattermann, K.; Müerköster, S.S.; Wedderkopp, H.; Knerlich-Lukoschus, F.; Ungefroren, H.; Mehdorn, H.M.; Mentlein, R. CX3CR1 Promotes Recruitment of Human Glioma-Infiltrating Microglia/Macrophages (GIMs). Exp. Cell Res. 2010, 316, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Laudati, E.; Currò, D.; Navarra, P.; Lisi, L. Blockade of CCR5 Receptor Prevents M2 Microglia Phenotype in a Microglia-Glioma Paradigm. Neurochem. Int. 2017, 108, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Arenas-Del Angel, M.C.; Zenteno, E.; Chávez, R.; Lascurain, R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 2009, 6, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, A.; Aucher, A.; Eissmann, P.; Vivier, E.; Davis, D.M. Membrane Nanotubes Facilitate Long-Distance Interactions between Natural Killer Cells and Target Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5545–5550. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, F.; Watzl, C. Impedance-Based Analysis of Natural Killer Cell Stimulation. Sci. Rep. 2018, 8, 4938. [Google Scholar] [CrossRef] [PubMed]
- Topham, N.J.; Hewitt, E.W. Natural Killer Cell Cytotoxicity: How Do They Pull the Trigger? Immunology 2009, 128, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M.; Dongre, P.; Hsu, H.-T.; Sinha, P.; James, A.M.; Mann, S.S.; Forbes, L.R.; Watkin, L.B.; Orange, J.S. Cell Biological Steps and Checkpoints in Accessing NK Cell Cytotoxicity. Immunol. Cell Biol. 2014, 92, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, A.; Dennert, G.; Singer, S.J. Polarization of the Golgi Apparatus and the Microtubule-Organizing Center within Cloned Natural Killer Cells Bound to Their Targets. Proc. Natl. Acad. Sci. USA 1983, 80, 7224–7228. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Martina, J.A.; Wu, X.S.; Hammer, J.A.; Long, E.O. Two Modes of Lytic Granule Fusion during Degranulation by Natural Killer Cells. Immunol. Cell Biol. 2011, 89, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.S.; Friedmann, K.S.; Mang, S.; Knörck, A.; Hoth, M.; Kummerow, C. Natural Killer Cells Induce Distinct Modes of Cancer Cell Death: Discrimination, Quantification, and Modulation of Apoptosis, Necrosis, and Mixed Forms. J. Biol. Chem. 2018, 293, 16348–16363. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. The Fas Signaling Pathway: More Than a Paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. TRAIL and Apoptosis Induction by TNF-Family Death Receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, J.P.; Spanholtz, J.; Tordoir, M.; Thijssen, V.L.; Heideman, D.A.M.; Verheul, H.M.W.; De Gruijl, T.D.; Van Der Vliet, H.J. Combination of NK Cells and Cetuximab to Enhance Anti-Tumor Responses in RAS Mutant Metastatic Colorectal Cancer. PLoS ONE 2016, 11, e0157830. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.-G.; Long, E.O. Synergy among Receptors on Resting NK Cells for the Activation of Natural Cytotoxicity and Cytokine Secretion. Blood 2006, 107, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Seidel, U.J.E.; Schlegel, P.; Lang, P. Natural Killer Cell Mediated Antibody-Dependent Cellular Cytotoxicity in Tumor Immunotherapy with Therapeutic Antibodies. Front. Immunol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Geller, M.A.; Miller, J.S. Use of Allogeneic NK Cells for Cancer Immunotherapy. Immunotherapy 2011, 3, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gangadaran, P.; Kalimuthu, S.; Oh, J.M.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Novel Alternatives to Extracellular Vesicle-Based Immunotherapy—Exosome Mimetics Derived from Natural Killer Cells. Artif. Cells Nanomed. Biotechnol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Melsen, J.E.; Lugthart, G.; Lankester, A.C.; Schilham, M.W. Human Circulating and Tissue-Resident CD56 bright Natural Killer Cell Populations. Front. Immunol. 2016, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK Cell CD16 Surface Expression and Function Is Regulated by a Disintegrin and Metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, S.; Eoli, M.; Frigerio, S.; Antozzi, C.; Bruzzone, M.G.; Cantini, G.; Nava, S.; Anghileri, E.; Cuppini, L.; Cuccarini, V.; et al. The Natural Killer Cell Response and Tumor Debulking Are Associated with Prolonged Survival in Recurrent Glioblastoma Patients Receiving Dendritic Cells Loaded with Autologous Tumor Lysates. Oncoimmunology 2013, 2, e23401. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, J.; Zimmer, J.; Chekenya, M. Natural Killer Cells in Intracranial Neoplasms: Presence and Therapeutic Efficacy against Brain Tumours. J. Neurooncol. 2014, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stringaris, K. Orphan NKs! The Mystery of the Self-Renewing NK Cells. Blood 2017, 129, 1890–1891. [Google Scholar] [CrossRef] [PubMed]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+and CD8+tumor-Infiltrating Immune Cells Correlate with Prolonged Survival in Glioblastoma Patients despite Integrated Immunosuppressive Mechanisms in the Tumor Microenvironment and at the Systemic Level. J. Neuroimmunol. 2013, 264, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mordoh, J.; Levy, E.M.; Roberti, M.P. Natural Killer Cells in Human Cancer: From Biological Functions to Clinical Applications. J. Biomed. Biotechnol. 2011, 2011, 676198. [Google Scholar]
- Farag, S.S. Natural Killer Cell Receptors: New Biology and Insights into the Graft-versus-Leukemia Effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling Natural Killer Cell Responses: Integration of Signals for Activation and Inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gras Navarro, A.; Kmiecik, J.; Leiss, L.; Zelkowski, M.; Engelsen, A.; Bruserud, Ø.; Zimmer, J.; Enger, P.Ø.; Chekenya, M. NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage To Efficiently Kill Glioblastoma and Prolong Animal Survival. J. Immunol. 2014, 193, 6192–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD155, an Onco-Immunologic Molecule in Human Tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Morales, M.; Sánchez-García, F.J.; Golán-Cancela, I.; Hernández-Pedro, N.; Costoya, J.A.; de la Cruz, V.P.; Moreno-Jiménez, S.; Sotelo, J.; Pineda, B. RB Mutation and RAS Overexpression Induce Resistance to NK Cell-Mediated Cytotoxicity in Glioma Cells. Cancer Cell Int. 2015, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Daga, A.; Dondero, A.; Zona, G.; Poliani, P.L.; Melotti, A.; Griffero, F.; Marubbi, D.; Spaziante, R.; Bellora, F.; et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol. 2009, 182, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Haspels, H.N.; Rahman, M.A.; Joseph, J.V.; Gras Navarro, A.; Chekenya, M. Glioblastoma Stem-Like Cells Are More Susceptible Than Differentiated Cells to Natural Killer Cell Lysis Mediated Through Killer Immunoglobulin-Like Receptors-Human Leukocyte Antigen Ligand Mismatch and Activation Receptor-Ligand Interactions. Front. Immunol. 2018, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D. The First Primary Brain-Tumor Operation. J. Neurosurg. 1984, 61, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Gzell, C.; Back, M.; Wheeler, H.; Bailey, D.; Foote, M. Radiotherapy in Glioblastoma: The Past, the Present and the Future. Clin. Oncol. 2017, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.P.; Recht, L.; Nagpal, S. Advances in the Management of Glioblastoma: The Role of Temozolomide and MGMT Testing. Clin. Pharmacol. Adv. Appl. 2013, 5, 1–9. [Google Scholar]
- Hashimoto, N. Cancer Immunotherapy for Gliomas: Overview and Future Directions. Neurol. Med. Chir. 2016, 56, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, H.J.G.; Peckham, M.J.; Richardson, A.E.; Alexander, P.A.; Payne, P.M. Glioblastoma Multiforme: A Controlled Trial to Assess the Value of Specific Active Immunotherapy in Patients Treated by Radical Surgery and Radiotherapy. Br. J. Cancer 1973, 27, 253–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir-Kaynak, E.; Qutub, A.A.; Yesil-Celiktas, O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front. Physiol. 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Rick, J.; Chandra, A.; Aghi, M.K. Tumor Treating Fields: A New Approach to Glioblastoma Therapy. J. Neurooncol. 2018, 137, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. Novo TTF-100A versus physician′s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Tsuboi, K.; Saijo, K.; Harada, H.; Takano, S.; Nose, T.; Ohno, T. Autologous Natural Killer Cell Therapy for Human Recurrent Malignant Glioma. Anticancer Res. 2004, 24, 1861–1871. [Google Scholar] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, K.; Khawja, S.; Ahmed, N. Adoptive Cell Therapies for Glioblastoma. Front. Oncol. 2013, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the Allogeneic Cell Line NK-92 in Patients with Advanced Renal Cell Cancer or Melanoma: A Phase I Trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Jachimowicz, R.D.; Fracasso, G.; Yazaki, P.J.; Power, B.E.; Borchmann, P.; Engert, A.; Hansen, H.P.; Reiners, K.S.; Marie, M.; von Strandmann, E.P.; et al. Induction of In Vitro and In Vivo NK Cell Cytotoxicity Using High-Avidity Immunoligands Targeting Prostate-Specific Membrane Antigen in Prostate Carcinoma. Mol. Cancer Ther. 2011, 10, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, D.J.; Hofmeister, C.; Padmanabhan, S.; Suvannasankha, A.; Jagannath, S.; Abonour, R.; Bakan, C.; Andre, P.; Efebera, Y.; Tiollier, J.; et al. A Phase 1 Trial of the Anti-KIR Antibody IPH2101 in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2012, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Höring, E.; Podlech, O.; Silkenstedt, B.; Rota, I.A.; Adamopoulou, E.; Naumann, U. The Histone Deacetylase Inhibitor Trichostatin a Promotes Apoptosis and Antitumor Immunity in Glioblastoma Cells. Anticancer Res. 2013, 33, 1351–1360. [Google Scholar] [PubMed]
- Kmiecik, J.; Navarro, A.G.; Poli, A.; Planagumà, J.; Zimmer, J.; Chekenya, M. Combining NK Cells and MAb9.2.27 to Combat NG2-Dependent and Anti-Inflammatory Signals in Glioblastoma. Oncoimmunology 2014, 3, e27185. [Google Scholar] [CrossRef] [PubMed]
- Yvon, E.S.; Burga, R.; Powell, A.; Cruz, C.R.; Fernandes, R.; Barese, C.; Nguyen, T.; Abdel-Baki, M.S.; Bollard, C.M. Cord Blood Natural Killer Cells Expressing a Dominant Negative TGF-β Receptor: Implications for Adoptive Immunotherapy for Glioblastoma. Cytotherapy 2017, 19, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric Antigen Receptor-Engineered NK-92 Cells: An off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Oh, J.M.; Gangadaran, P.; Kalimuthu, S.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Targeting and Therapy of Glioblastoma in a Mouse Model Using Exosomes Derived From Natural Killer Cells. Front. Immunol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Nakazawa, T.; Natsume, A.; Nishimura, F.; Nakamura, M.; Matsuda, R.; Omoto, K.; Tanaka, Y.; Shida, Y.; Park, Y.; et al. Novel Human NK Cell Line Carrying CAR Targeting EGFRvIII Induces Antitumor Effects in Glioblastoma Cells. Anticancer Res. 2018, 38, 5049–5505. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kang, W.Y.; Yoon, Y.; Jin, J.Y.; Song, H.J.; Her, J.H.; Kang, S.M.; Hwang, Y.K.; Kang, K.J.; Joo, K.M.; et al. Natural Killer (NK) Cells Inhibit Systemic Metastasis of Glioblastoma Cells and Have Therapeutic Effects against Glioblastomas in the Brain. BMC Cancer 2015, 15, 1011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mahajan, B.B.; Kaur, S.; Singh, A. Autologous Therapies in Dermatology. J. Clin. Aesthet. Dermatol. 2014, 7, 38–45. [Google Scholar] [PubMed]
- Kazmi, B.; Inglefield, C.J.; Lewis, M.P. Autologous Cell Therapy: Current Treatments and Future Prospects. Wounds 2009, 21, 234–242. [Google Scholar] [PubMed]
- Jacobs, S.K.; Wilson, D.J.; Kornblith, P.L.; Grimm, E.A. In Vitro Killing of Human Glioblastoma by Interleukin-2- Activated Autologous Lymphocytes. Cancer Res. 1986, 46, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; Schulman, I.H.; Balkan, W.; Hare, J.M. Allogeneic Cell Therapy. Circ. Res. 2015, 116, 12–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, O.; Jung, M.Y.; Hwang, Y.K.; Shin, E.C. Present and Future of Allogeneic Natural Killer Cell Therapy. Front. Immunol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G.; Valiante, N. Receptors for the Fc Fragment of IgG on Natural Killer Cells. Nat. Immun. 1993, 12, 218–234. [Google Scholar] [PubMed]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Exosomes Derived from Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef] [PubMed]


| Year | Events | Ref. |
|---|---|---|
| 1884 | First recognized surgery for primary brain tumor resection | [56] |
| 1940s | Implementation of radiotherapy in the treatment of brain tumors | [57] |
| 1950s | First chemotherapy session | [58] |
| 1970s | Incorporation of computed tomography (CT) into radiotherapy planning | [57] |
| Synthesis of TMZ | [2,58] | |
| Emergence of immunotherapy against gliomas concept | [59,60] | |
| 1980s | Incorporation of magnetic resonance imaging (MRI) | [57] |
| 1993 | It is discovered that combination of chemotherapy with radiotherapy increases patients survival | [61] |
| Nowadays | Gold standard therapy for GB patients-surgery in combination with radiotherapy and/or chemotherapy | [61] |
| FDA has approved TMZ like chemotherapeutic agent for GB patients | [2] | |
| Development of new strategies: | ||
| Tumor treating fields (TTF): application of low intensity electric fields on brain tumors. FDA has approved the use of TTF in early diagnosis and recurrent GB patients | [62,63] | |
| New disvoveries in the use of nanosystems against GB | ||
| Advances in NK cell-based immunotherapy: | ||
| 2002 | The use of allogeneic NK cells expressing KIR receptors which are not able to recognize the patient’s MHC-class I molecules | [39,64] |
| 2004 | Autologous NK cell therapy for recurrent GB | [65] |
| 2005 | The use of cord blood as a source of NK cells | [66] |
| 2006 | Phase II trial: intralesional autologous LAK cells therapy in recurrent GB patients. This study has been completed in 2013 | [67] |
| 2008 | Phase I trial: allogeneic NK-92 cell line therapy in patients with advanced renal cell cancer or melanoma | [68] |
| 2011 | Induction of NK cell cytotoxicity using a immunoligand conjugated to a NKG2D receptor that recognizes tumor specific antigens. This approach has been tested in prostate carcinoma cell lines | [69] |
| 2012 | Phase I trial: therapy with an anti-KIR antibody in multiple myeloma patients. This antibody blocks the binding of KIR with MHC-class I molecules and avoids the inhibitory response of NK cells | [70] |
| 2013 | Combination of NK cells with Trichostatin A induces MICA expression on GB cells allowing to increase NK cytotoxicity | [71] |
| 2014 | Combination of NKs with mAb9.2.27 antibody changes the GB anti-Inflammatory microenvironment into a pro-inflammatory one | [72] |
| Identification of KIR2DS2 as a potent activating receptor on alloreactive NK cells which decreases cell proliferation and angiogenesis GB tumors | [51] | |
| 2016 | Apoptosis mediated by CD16 receptors after the recognition of antibodies, such as cetuximab, which are blocking EGFR on tumor cells. This approach has been tested in colorectal cancer cell lines | [36] |
| 2017 | Cord blood-derived NK cells expressing a dominant negative TGF-β receptor II (DNRII) recognize and kill GB tumor cells even in the presence of TGF-β | [73] |
| Phase I trial: intracranial injection of ErbB2-specific NK-92/5.28.z (HER2.taNK) cells in patients with recurrent HER2-positive GB | [74,75] | |
| 2018 | Study of NK cells-derived exosomes as potential immunotherapeutic agents for cancer treatment | [76] |
| Establishment of a chimeric antigen receptor (CAR) on NK cell line which recognizes EGFRvIII and promotes apoptosis in GB cells | [77] | |
| Preclinical studies; Clinical studies | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golán, I.; Rodríguez de la Fuente, L.; Costoya, J.A. NK Cell-Based Glioblastoma Immunotherapy. Cancers 2018, 10, 522. https://doi.org/10.3390/cancers10120522
Golán I, Rodríguez de la Fuente L, Costoya JA. NK Cell-Based Glioblastoma Immunotherapy. Cancers. 2018; 10(12):522. https://doi.org/10.3390/cancers10120522
Chicago/Turabian StyleGolán, Irene, Laura Rodríguez de la Fuente, and Jose A. Costoya. 2018. "NK Cell-Based Glioblastoma Immunotherapy" Cancers 10, no. 12: 522. https://doi.org/10.3390/cancers10120522