Anticancer Activity of Cynomorium coccineum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Cynomorium Plants
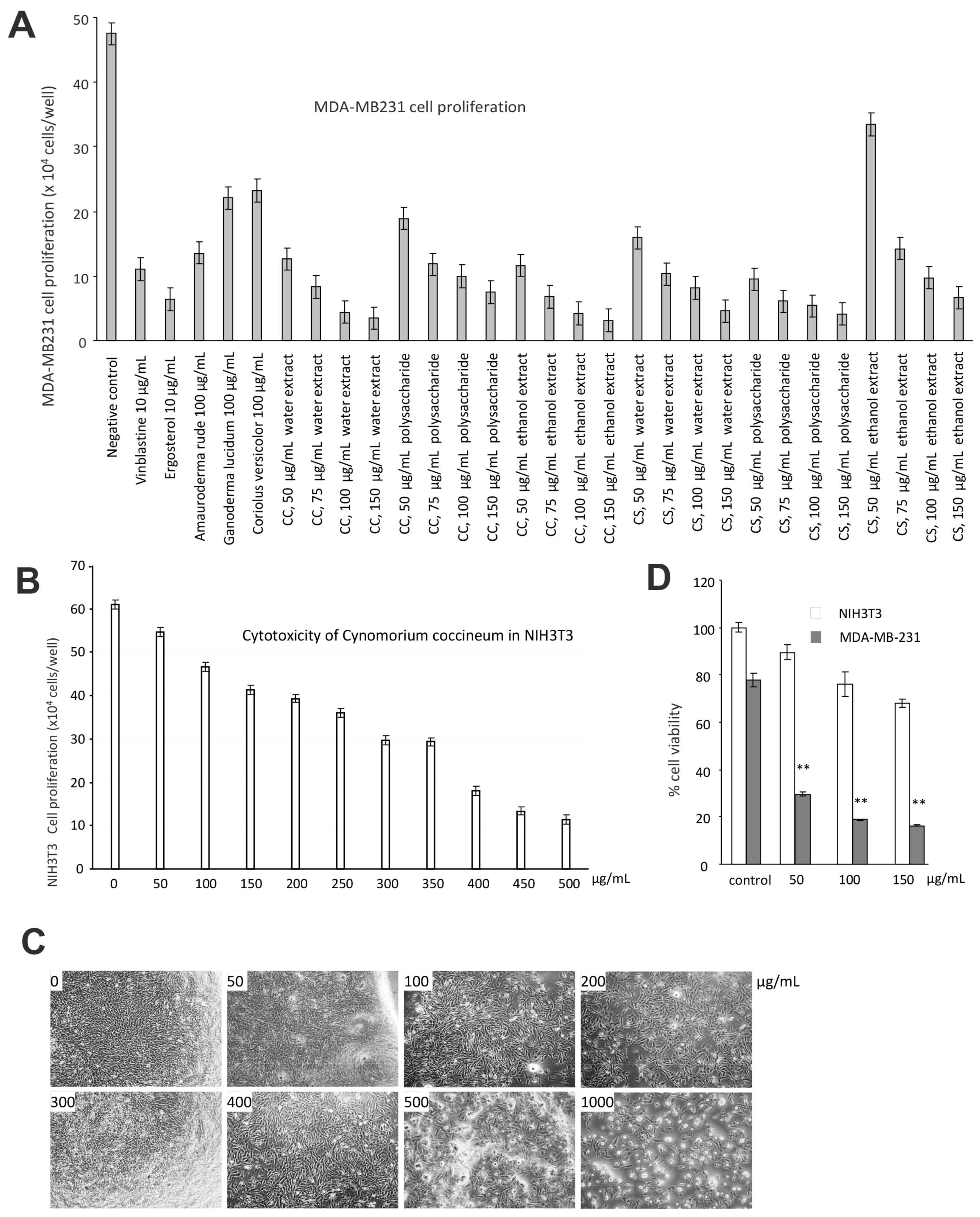
2.3. Cell Proliferation Assay
2.4. Cell Migration
2.5. Cell Invasion
2.6. Cell Survival
2.7. Cell Cycle Analysis
2.8. Colony Formation
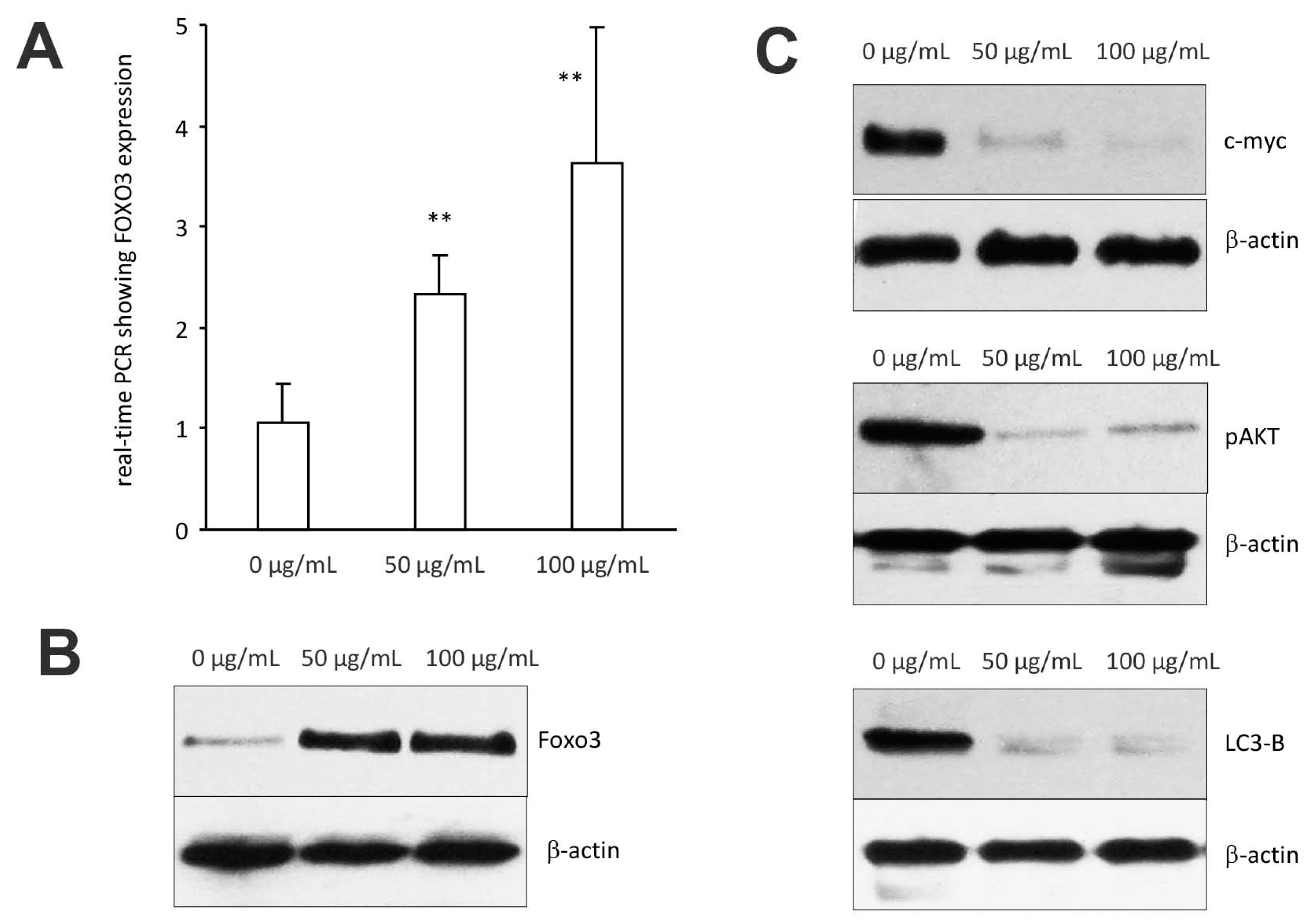
2.9. Western Blot
2.10. Real-Time PCR
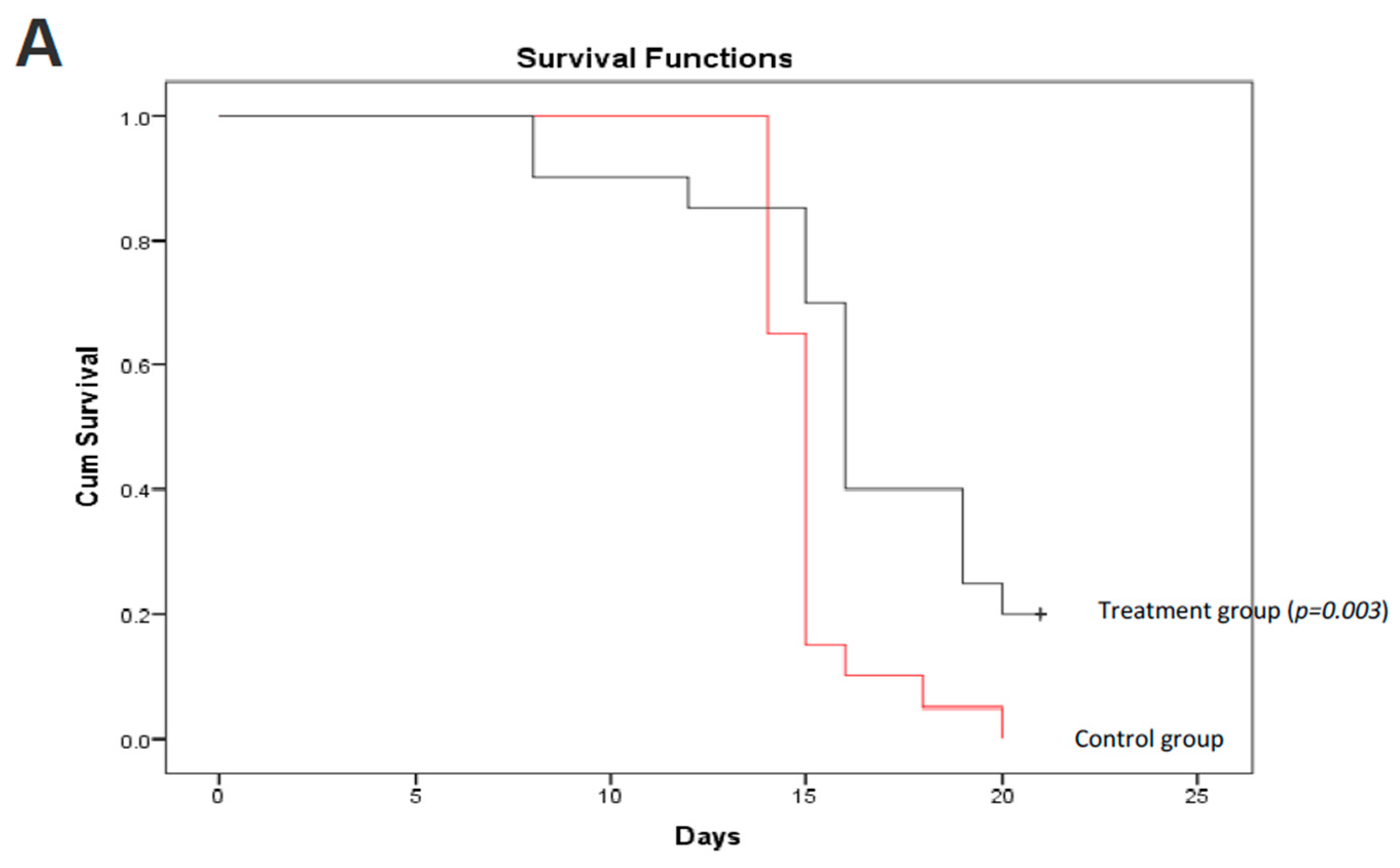
2.11. Animal Survival Experiment
3. Results and Discussion
3.1. Cynomorium Coccineum Induces Cancer Cell Death by Modulating Cell Cycle Progression
3.2. Cynomorium Coccineum Inhibits Cancer Cell Migration, Invasion, and Colony Formation
3.3. Cynomorium Coccineum Treatment Prolonged Animal Survival
3.4. Regulation of Signal Protein Expression by Cynomorium Coccineum
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s modified Eagle’s medium: |
| FBS | fetal bovine serum; |
| PAGE | Polyacrylamide gel electrophoresis. |
References
- Liu, T.; Men, Q.; Wu, G.; Yu, C.; Huang, Z.; Liu, X.; Li, W. Tetrandrine induces autophagy and differentiation by activating ROS and Notch1 signaling in leukemia cells. Oncotarget 2015, 6, 7992–8006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, V.K.; Chiu, P.; Chung, S.S.; Chow, L.M.; Zhao, Y.Z.; Yang, B.B.; Ko, B.C. Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo. Clin. Cancer Res. 2005, 11, 6002–6011. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Govindarajan, R. Cancer—An ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, C.; Ahmad, B.; Huang, L. Optimization of Extract Method for Cynomorium songaricum Rupr. by Response Surface Methodology. J. Anal. Methods Chem. 2017, 2017, 6153802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wang, X.; Zhang, G.; Wang, Y.; Di, D. Evaluation of the free radical scavenging activity of Cynomorium songaricum Rupr. by a novel DPPH-HPLC method. J. Food Sci. 2011, 76, C1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, H.A.; Kwon, J.Y.; Jeong, M.H.; Lee, J.S.; Kang, D.W.; Choi, D. The Effects of Cynomorium songaricum on the Reproductive Activity in Male Golden Hamsters. Dev. Reprod. 2013, 17, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.M.; Kim, H.Y.; Park, S.Y.; Kim, H.M.; Chang, M.S.; Park, S.K. Cynomorium songaricum induces spermatogenesis with glial cell-derived neurotrophic factor (GDNF) enhancement in rat testes. J. Ethnopharmacol. 2010, 128, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Dar, M.S.; Fakouhi, T. Hypotensive agent from Cynomorium coccineum. Pahlavi Med. J. 1978, 9, 167–181. [Google Scholar] [PubMed]
- Al-Qarawi, A.A.; Abdel-Rahman, H.A.; El-Badry, A.A.; Harraz, F.; Razig, N.A.; Abdel-Magied, E.M. The effect of extracts of Cynomorium coccineum and Withania somnifera on gonadotrophins and ovarian follicles of immature Wistar rats. Phytother. Res. 2000, 14, 288–290. [Google Scholar] [CrossRef]
- Goncalves, M.J.; Piras, A.; Porcedda, S.; Marongiu, B.; Falconieri, D.; Cavaleiro, C.; Rescigno, A.; Rosa, A.; Salgueiro, L. Antifungal activity of extracts from Cynomorium coccineum growing wild in Sardinia island (Italy). Nat. Prod. Res. 2015, 29, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, R.; Yang, J.; Miao, L.; Wang, Y.; Munyangaju, J.E.; Wichai, N.; Wang, H.; Zhu, Y.; Liu, E.; et al. Compounds from Cynomorium songaricum with Estrogenic and Androgenic Activities Suppress the Oestrogen/Androgen-Induced BPH Process. Evid.-Based Complement. Altern. Med. 2017, 2017, 6438013. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Q.; Wang, W.; Li, X.; Zhang, J. A polysaccharide isolated from Cynomorium songaricum Rupr. protects PC12 cells against H2O2-induced injury. Int. J. Biol. Macromol. 2016, 87, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Nakamura, N.; Miyashiro, H.; Hattori, M.; Shimotohno, K. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem. Pharm. Bull. 1999, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, G.G.; Rong, P.; Zhang, Z.; Dong, J.; Zhao, H.; Li, H.; Li, Y.; Pan, J.; Liu, H.; et al. Therapeutic effects of radix dipsaci, pyrola herb, and Cynomorium songaricum on bone metabolism of ovariectomized rats. BMC Complement. Altern. Med. 2012, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Choi, J.H.; Kim, W.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Yoon, Y.S.; Yoo, K.Y.; Won, M.H.; Hwang, I.K. Cynomorium songaricum extract enhances novel object recognition, cell proliferation and neuroblast differentiation in the mice via improving hippocampal environment. BMC Complement. Altern. Med. 2014, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Du, W.W.; Li, X.; Yee, A.J.; Yang, B.B. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene 2016, 35, 3919–3931. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Du, W.W.; Wu, N.; Yang, W.; Awan, F.M.; Fang, L.; Ma, J.; Li, X.; Zeng, Y.; Yang, Z.; et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Diff. 2017, 24, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics 2017, 7, 3842–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.G.; Awan, F.M.; Du, W.W.; Zeng, Y.; Lyu, J.; Wu, D.; Gupta, S.; Yang, W.; Yang, B.B. The Circular RNA Interacts with STAT3, Increasing Its Nuclear Translocation and Wound Repair by Modulating Dnmt3a and miR-17 Function. Mol. Ther. 2017, 25, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Diff. 2017, 24, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Du, W.W.; Lyu, J.; Dong, J.; Zhang, C.; Yang, W.; He, A.; Kwok, Y.S.S.; Ma, J.; Wu, N.; et al. Enhanced breast cancer progression by mutant p53 is inhibited by the circular RNA circ-Ccnb1. Cell Death Diff. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abd el-Rahman, H.A.; el-Badry, A.A.; Mahmoud, O.M.; Harraz, F.A. The effect of the aqueous extract of Cynomorium coccineum on the epididymal sperm pattern of the rat. Phytother. Res. 1999, 13, 248–250. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nicholson, E.H.; Nickrent, D.L. Extensive intraindividual variation in plastid rDNA sequences from the holoparasite Cynomorium coccineum (Cynomoriaceae). J. Mol. Evol. 2004, 58, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, E.M.; Abdel-Rahman, H.A.; Harraz, F.M. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J. Ethnopharmacol. 2001, 75, 1–4. [Google Scholar] [CrossRef]
- Rosa, A.; Rescigno, A.; Piras, A.; Atzeri, A.; Scano, P.; Porcedda, S.; Zucca, P.; Assunta Dessi, M. Chemical composition and effect on intestinal Caco-2 cell viability and lipid profile of fixed oil from Cynomorium coccineum L. Food Chem. Toxicol. 2012, 50, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Nieddu, M.; Piras, A.; Atzeri, A.; Putzu, D.; Rescigno, A. Maltese mushroom (Cynomorium coccineum L.) as source of oil with potential anticancer activity. Nutrients 2015, 7, 849–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, P.; Rosa, A.; Tuberoso, C.I.; Piras, A.; Rinaldi, A.C.; Sanjust, E.; Dessi, M.A.; Rescigno, A. Evaluation of antioxidant potential of “maltese mushroom” (Cynomorium coccineum) by means of multiple chemical and biological assays. Nutrients 2013, 5, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.; Xie, Y.Z.; Yang, X.; Li, H.; Li, X.M.; Pan, H.H.; Cai, M.H.; Zhong, H.M.; Yang, B.B. Anticancer activity of Amauroderma rude. PLoS ONE 2013, 8, e66504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Q.; Bu, M.; Hu, L.; Du, W.W.; Jiao, C.; Pan, H.; Sdiri, M.; Wu, N.; Xie, Y.; et al. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 2016, 7, 33948–33959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.P.; Xie, Y.Z.; Deng, Z.; Li, X.M.; Yang, W.; Jiao, C.W.; Fang, L.; Li, S.Z.; Pan, H.H.; Yee, A.J.; et al. Ergosterol peroxide isolated from Ganoderma lucidum abolishes microRNA miR-378-mediated tumor cells on chemoresistance. PLoS ONE 2012, 7, e44579. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Yong, T.; Wang, Z.; Su, J.; Jiao, C.; Xie, Y.; Yang, B.B. Cytotoxic lanostane-type triterpenoids from the fruiting bodies of Ganoderma lucidum and their structure-activity relationships. Oncotarget 2017, 8, 10071–10084. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Dhaliwal, P.; Xie, Y.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, I.B.; Zucca, P.; Marincola, F.C.; Piras, A.; Rosa, A.; Chaieb, M.; Rescigno, A. Chemical Composition and Antioxidant Potential Differences between Cynomorium coccineum L. Growing in Italy and in Tunisia: Effect of Environmental Stress. Diversity 2018, 10, 53. [Google Scholar] [CrossRef]
- Zucca, P.; Argiolas, A.; Nieddu, M.; Pintus, M.; Rosa, A.; Sanna, F.; Sollai, F.; Steri, D.; Rescigno, A. Biological Activities and Nutraceutical Potentials of Water Extracts from Different Parts of Cynomorium coccineum L. (Maltese Mushroom). Pol. J. Food Nutr. Sci. 2016, 66, 179–188. [Google Scholar] [CrossRef] [Green Version]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdiri, M.; Li, X.; Du, W.W.; El-Bok, S.; Xie, Y.-Z.; Ben-Attia, M.; Yang, B.B. Anticancer Activity of Cynomorium coccineum. Cancers 2018, 10, 354. https://doi.org/10.3390/cancers10100354
Sdiri M, Li X, Du WW, El-Bok S, Xie Y-Z, Ben-Attia M, Yang BB. Anticancer Activity of Cynomorium coccineum. Cancers. 2018; 10(10):354. https://doi.org/10.3390/cancers10100354
Chicago/Turabian StyleSdiri, Mouna, Xiangmin Li, William W. Du, Safia El-Bok, Yi-Zhen Xie, Mossadok Ben-Attia, and Burton B. Yang. 2018. "Anticancer Activity of Cynomorium coccineum" Cancers 10, no. 10: 354. https://doi.org/10.3390/cancers10100354




