Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models
Abstract
:1. Introduction
2. Results
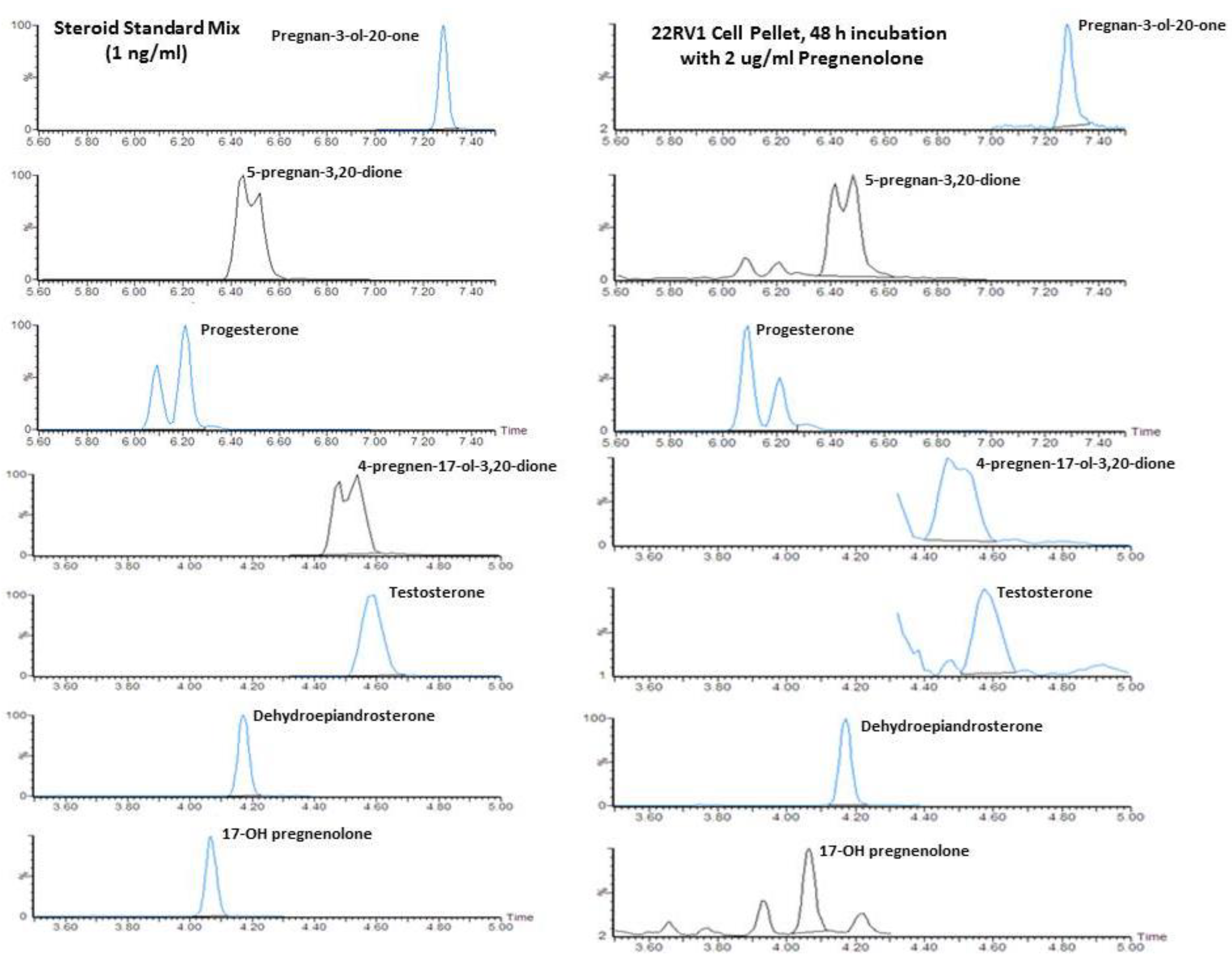
2.1. Quantification of Steroid Formation by LC/MS
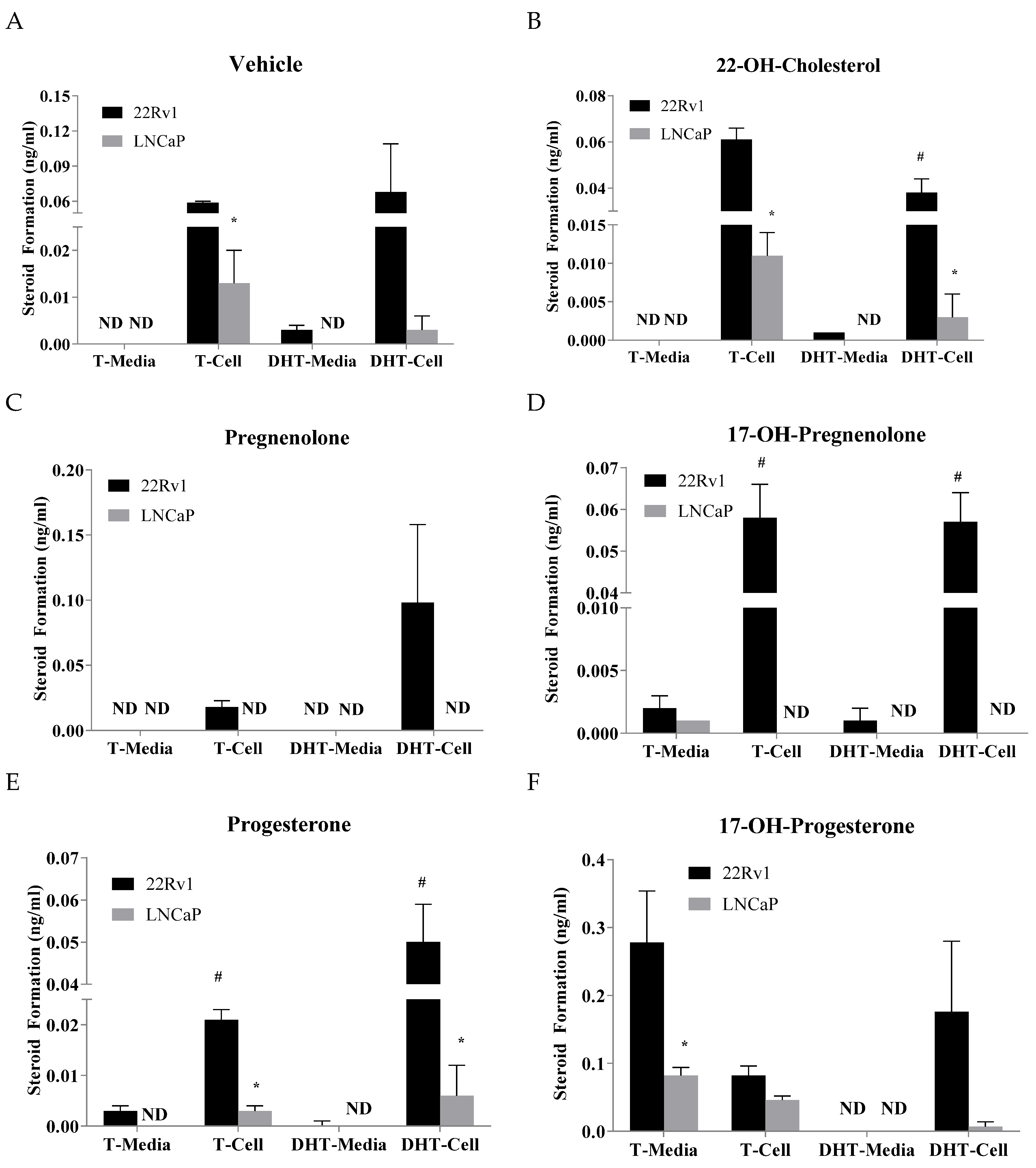
2.2. Effects of Precursor Stimulation on the Steroidogenesis Pathway in the In Vitro Models
2.3. Effects of Precursors on Steroidogenic Enzymes in the In Vitro Models
2.4. Effects of 17-OH-Pregnenolone and Progesterone on Steroid Metabolism in Human Peripheral Zone Prostate Tissue
3. Discussion
4. Materials and Methods
4.1. Standards, Chemicals and Reagents
4.2. Growth and Treatment of Human Cell Lines
4.3. Human Prostate Cancer Tissues
4.4. Steroid Extraction and Derivatization Reactions
4.5. Analysis of Steroids by LC-MS
4.6. Steroid Biotransformation Assay with Human Prostate Homogenates
4.7. mRNA Expression in Prostate Cancer Cells
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fluck, C.E.; Pandey, A.V. Steroidogenesis of the testis-new genes and pathways. Ann. Endocrinol. (Paris) 2014, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, J.A.; Guns, E.S.; Lubik, A.A.; Adomat, H.H.; Hendy, S.C.; Wood, C.A.; Ettinger, S.L.; Gleave, M.E.; Nelson, C.C. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008, 68, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.A.; Nelson, C.C.; Adomat, H.H.; Hendy, S.C.; Hendy, S.C.; Gleave, M.E.; Guns, E.S. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J. Steroid Biochem. Mol. Biol. 2009, 115, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Duff, J.; McEwan, I.J. Mutation of histidine 874 in the androgen receptor ligand-binding domain leads to promiscuous ligand activation and altered p160 coactivator interactions. Mol. Endocrinol. 2005, 19, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E.; et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Castillo-Martin, M.; Puzio-Kuter, A.; Yan, J.; Foster, T.H.; Gao, H.; Sun, Y.; Ouyang, X.; Gerald, W.L.; Cordon-Cardo, C.; et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J. Clin. Investig. 2008, 118, 3051–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, D.A.; Witte, O.N. Stem cells in prostate cancer initiation and progression. J. Clin. Investig. 2007, 117, 2044–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, N.C.; Hooper, J.D.; Lambie, D.; Lee, C.S.; Yang, T.; Vesey, D.A.; Samaratunga, H.; Johnson, D.W.; Gobe, G.C. Evidence for steroidogenic potential in human prostate cell lines and tissues. Am. J. Pathol. 2012, 181, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, S.; Ng, P.; Bubley, G.J.; Nelson, P.S.; Mostaghel, E.A.; Marck, B.; Matsumoto, A.M.; Simon, N.I.; Wang, H.; et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011, 71, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, N.; Sung, C.C.; Schultz, N.; Danila, D.C.; He, B.; Eedunuri, V.K.; Fleisher, M.; Sander, C.; Sawyers, C.L.; Scher, H.I. Distinct Patterns of Dysregulated Expression of Enzymes Involved in Androgen Synthesis and Metabolism in Metastatic Prostate Cancer Tumors. Cancer Res. 2012, 72, 6142–6152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu-The, V. Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Titus, M.A.; Wilson, E.M. Potential prostate cancer drug target: Bioactivation of androstanediol by conversion to dihydrotestosterone. Clin. Cancer Res. 2011, 17, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, M.; Emamekhoo, H.; Park, S.; Taylor, J.; Almassi, N.; Upadhyay, S.; Tyler, A.; Berk, M.P.; Hu, B.; Hwang, T.H.; et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J. Clin. Investig. 2018, 128, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Li, R.; Papari-Zareei, M.; Watumull, L.; Zhao, Y.D.; Auchus, R.J.; Sharifi, N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 13728–13733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.J.; Smit, F.P.; Sedelaar, J.P.; Schalken, J.A. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol. Med. 2011, 17, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP Model of Human Prostatic Carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Sobel, R.E.; Sadar, M.D. Cell lines used in prostate cancer research: A compendium of old and new lines-part 1. J. Urol. 2005, 173, 342–359. [Google Scholar] [CrossRef] [PubMed]
- Dillard, P.R.; Lin, M.F.; Khan, S.A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol. Cell. Endocrinol. 2008, 295, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofland, J.; van Weerden, W.M.; Dits, N.F.; Steenbergen, J.; van Leenders, G.J.; Jenster, G.; Schröder, F.H.; de Jong, F.H. Evidence of Limited Contributions for Intratumoral Steroidogenesis in Prostate Cancer. Cancer Res. 2010, 70, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubik, A.A.; Gunter, J.H.; Hendy, S.C.; Locke, J.A.; Adomat, H.H.; Thompson, V.; Herington, A.; Gleave, M.E.; Pollak, M.; Nelson, C.C. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011, 71, 5754–5764. [Google Scholar] [CrossRef] [PubMed]
- Sramkoski, R.M.; Pretlow, T.G.; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.; Sadar, M.D. Androgen receptor and its splice variants in prostate cancer. Cell. Mol. Life Sci. 2011, 68, 3971–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jae, Y.C.; Nadiminty, N.; Dutt, S.; Lou, W.; Yang, J.C.; Kung, H.J.; Evans, C.P.; Gao, A.C. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin. Cancer Res. 2009, 15, 4815–4822. [Google Scholar]
- Pham, S.; Deb, S.; Ming, D.S.; Adomat, H.; Hosseini-Beheshti, E.; Zoubeidi, A.; Gleave, M.; Guns, E.S. Next-generation steroidogenesis inhibitors, dutasteride and abiraterone, attenuate but still do not eliminate androgen biosynthesis in 22RV1 cells in vitro. J. Steroid Biochem. Mol. Biol. 2014, 144PB, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.S.; Pham, S.; Deb, S.; Chin, M.Y.; Kharmate, G.; Adomat, H.; Beheshti, E.H.; Locke, J.; Guns, E.T. Pomegranate extracts impact the androgen biosynthesis pathways in prostate cancer models in vitro and in vivo. J. Steroid Biochem. Mol. Biol. 2014, 143, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Attardi, B.J.; Burgenson, J.; Hild, S.A.; Reel, J.R. Steroid hormonal regulation of growth, prostate specific antigen secretion, and transcription mediated by the mutated androgen receptor in CWR22Rv1 human prostate carcinoma cells. Mol. Cell. Endocrinol. 2004, 222, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.S.; Kish, K.F.; Wang, C.; Attar, R.M.; Kiefer, S.E.; An, Y.; Wu, G.Y.; Scheffler, J.E.; Salvati, M.E.; Krystek, S.R., Jr.; et al. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc. Natl. Acad. Sci. USA 2001, 98, 4904–4909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steketee, K.; Timmerman, L.; Ziel-van der Made, A.C.; Doesburg, P.; Brinkmann, A.O.; Trapman, J. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int. J. Cancer 2002, 100, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, J.T.; Le, H.; McFann, K.K.; Blackman, M.R. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E573–E584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.A.; Sharief, Y.; Hamil, K.G.; Gregory, C.W.; Zang, D.Y.; Sar, M.; Gumerlock, P.H.; deVere White, R.W.; Pretlow, T.G.; Harris, S.E.; et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol. Endocrinol. 1997, 11, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, F.; Nishiyama, T.; Kawasaki, T.; Miyashiro, Y.; Hara, N.; Takizawa, I.; Naito, M.; Takahashi, K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013, 3, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, C.W.; Yoon, C.Y.; Jeong, S.J.; Hong, S.K.; Byun, S.S.; Lee, S.E. Limited Expression of Cytochrome P450 17α-Hydroxylase/17,20-Lyase in Prostate Cancer Cell Lines. Korean J. Urol. 2011, 52, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Q.; Liu, S.V.; Montgomery, R.B.; Stanczyk, F.Z.; Vallone, J.G.; Merin, N.M.; Pinski, J. Effects of luteinizing hormone receptor signaling in prostate cancer cells. Prostate 2015, 75, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.M.; Samara, E.N.; Wong, C.; Metwalli, A.; Krlin, R.; Bane, B.; Liu, C.Z.; Yang, J.T.; Pitha, J.V.; Culkin, D.J.; et al. Increased expression of type 2 3-hydroxysteroid dehydrogenase/type 5 17 -hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr. Relat. Cancer 2006, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lubik, A.A.; Gunter, J.H.; Hollier, B.G.; Ettinger, S.; Fazli, L.; Stylianou, N.; Hendy, S.C.; Adomat, H.H.; Gleave, M.E.; Pollak, M.; et al. IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocr. Relat. Cancer 2013, 20, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri-Cesi, P.; Poletti, A.; Colciago, A.; Magni, P.; Martini, P.; Motta, M. Presence of 5alpha-reductase isozymes and aromatase in human prostate cancer cells and in benign prostate hyperplastic tissue. Prostate 1998, 34, 283–291. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Cai, L.Q.; You, X.; Cordero, J.J.; Huang, Y.; Imperato-McGinley, J. Androgen-induced prostate-specific antigen gene expression is mediated via dihydrotestosterone in LNCaP cells. J. Androl. 2003, 24, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, L.; Wang, Y.; Zhang, H.; Xu, D.; Zhao, X.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar] [PubMed]
- Tomlinson Guns, E.S.; University of British Columbia, Vancouver, BC, Canada. Personal communication, 2018.
- Castagnetta, L.; Granata, O.M.; Polito, L.; Blasi, L.; Cannella, S.; Carruba, G. Different conversion metabolic rates of testosterone are associated to hormone-sensitive status and-response of human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 1994, 49, 351–357. [Google Scholar] [CrossRef]
- Titus, M.A.; Li, Y.; Kozyreva, O.G.; Maher, V.; Godoy, A.; Smith, G.J.; Mohler, J.L. 5α-Reductase Type 3 Enzyme in Benign and Malignant Prostate. Prostate 2014, 74, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Tamura, K.; Chung, S.; Honma, S.; Okuyama, A.; Nakamura, Y.; Nakagawa, H. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci. 2008, 99, 81–86. [Google Scholar] [PubMed]
- Li, J.; Ding, Z.; Wang, Z.; Lu, J.F.; Maity, S.N.; Navone, N.M.; Logothetis, C.J.; Mills, G.B.; Kim, J. Androgen regulation of 5alpha-reductase isoenzymes in prostate cancer: Implications for prostate cancer prevention. PLoS ONE 2011, 6, e28840. [Google Scholar] [CrossRef] [PubMed]



| 22RV1 | Vehicle | 22-OH-Cholesterol | Pregnenolone | Progesterone | 17-OH-Pregnenolone | 17-OH-Progesterone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Pellet | Avg | SEM | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p |
| Dehydroepiandrosterone | 0.076 | 0.020 | 0.077 | 0.002 | 1.261 | 0.213 | * | 0.292 | 0.043 | * | 15.674 | 6.606 | 0.146 | 0.021 | |||
| Androstenedione | 0.023 | 0.001 | 0.013 | 0.003 | * | 0.029 | 0.013 | 0.327 | 0.179 | 0.022 | 0.008 | 0.510 | 0.183 | ||||
| 17-OH-Progesterone | 0.223 | 0.110 | 0.249 | 0.219 | 0.078 | 0.046 | 0.994 | 0.094 | * | 6.188 | 0.393 | * | 374.535 | 19.418 | * | ||
| Testosterone | 0.059 | 0.001 | 0.061 | 0.005 | 0.018 | 0.005 | * | 0.021 | 0.002 | * | 0.058 | 0.008 | 0.082 | 0.014 | |||
| Dihydrotestosterone | 0.068 | 0.041 | 0.038 | 0.006 | 0.098 | 0.060 | 0.050 | 0.009 | 0.057 | 0.007 | 0.176 | 0.104 | |||||
| Androsterone | 0.002 | 0.001 | 0.002 | 0.001 | 0.015 | 0.010 | 0.029 | 0.015 | 0.006 | 0.001 | 0.295 | 0.016 | * | ||||
| 5-Pregnan-3,17-diol-20-one | 0.000 | 0.000 | 0.012 | 0.012 | 0.000 | 0.000 | 0.156 | 0.127 | 0.295 | 0.028 | * | 101.551 | 29.255 | * | |||
| Pregnenolone | 5.249 | 1.113 | 7.483 | 0.258 | 1284.503 | 401.638 | * | 268.962 | 11.452 | * | 5.076 | 0.682 | 9.375 | 0.099 | * | ||
| Progesterone | 0.264 | 0.123 | 0.801 | 0.361 | 16.099 | 1.930 | * | 620.243 | 88.930 | * | 0.108 | 0.056 | 0.255 | 0.082 | |||
| 5-Pregnan-3,20-dione | 0.011 | 0.006 | 0.048 | 0.044 | 1.763 | 0.362 | * | 175.921 | 20.090 | * | 0.006 | 0.006 | 0.022 | 0.012 | |||
| 17-OH-pregnenolone | 0.515 | 0.138 | 0.762 | 0.060 | 14.725 | 3.315 | * | 0.590 | 0.205 | 2917.082 | 867.097 | * | 1.266 | 0.376 | |||
| 5-Pregnan-3-ol-20-one | 0.002 | 0.001 | 0.004 | 0.004 | 0.626 | 0.109 | * | 107.079 | 5.560 | * | 0.001 | 0.000 | 0.040 | 0.015 | |||
| 5-Pregnan-17-ol-3,20-dione | 0.000 | 0.000 | 0.009 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.145 | 0.032 | * | 17.217 | 3.558 | * | |||
| 5a-Androstan-3,17-dione | 0.030 | 0.023 | 0.007 | 0.007 | 0.000 | 0.000 | 0.054 | 0.042 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| LNCaP | Vehicle | 22-OH-Cholesterol | Pregnenolone | Progesterone | 17-OH-Pregnenolone | 17-OH-Progesterone | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Pellet | Avg | SEM | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p | Avg | SEM | p | |
| Dehydroepiandrosterone | 0.127 | 0.051 | 0.188 | 0.026 | 0.254 | 0.026 | 0.261 | 0.036 | 2.398 | 0.093 | * | 0.251 | 0.007 | |||||
| Androstenedione | 0.000 | 0.000 | 0.010 | 0.010 | 0.000 | 0.000 | 0.008 | 0.008 | 0.041 | 0.002 | 0.278 | 0.023 | ||||||
| 17-OH-Progesterone | 0.000 | 0.000 | 0.036 | 0.018 | 0.000 | 0.000 | 0.000 | 0.000 | 7.649 | 1.122 | * | 91.159 | 11.527 | * | ||||
| Testosterone | 0.013 | 0.007 | 0.011 | 0.003 | 0.000 | 0.000 | 0.003 | 0.001 | 0.000 | 0.000 | 0.046 | 0.006 | * | |||||
| Dihydrotestosterone | 0.003 | 0.003 | 0.003 | 0.003 | 0.000 | 0.000 | 0.006 | 0.006 | 0.000 | 0.000 | 0.007 | 0.007 | ||||||
| Androsterone | 0.041 | 0.011 | 0.000 | 0.000 | * | 0.000 | 0.000 | * | 0.020 | 0.020 | 0.023 | 0.023 | 0.814 | 0.063 | * | |||
| 5-Pregnan-3,17-diol-20-one | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.004 | 0.000 | 0.000 | 0.514 | 0.055 | * | 45.665 | 3.027 | * | ||||
| Pregnenolone | 3.946 | 1.045 | 5.155 | 0.220 | 1505.414 | 135.597 | * | 406.659 | 35.203 | * | 4.691 | 0.228 | 9.830 | 0.607 | * | |||
| Progesterone | 0.000 | 0.000 | 0.000 | 0.000 | 4.596 | 0.289 | * | 26.695 | 4.022 | * | 0.063 | 0.006 | * | 0.026 | 0.026 | |||
| 5-Pregnan-3,20-dione | 0.036 | 0.009 | 0.039 | 0.009 | 0.780 | 0.075 | * | 34.105 | 4.286 | * | 0.031 | 0.008 | 0.036 | 0.007 | ||||
| 17-OH-pregnenolone | 0.008 | 0.008 | 0.000 | 0.000 | 0.516 | 0.082 | * | 0.016 | 0.016 | 512.809 | 19.425 | * | 0.160 | 0.013 | * | |||
| 5-Pregnan-3-ol-20-one | 0.000 | 0.000 | 0.000 | 0.000 | 0.391 | 0.072 | * | 63.744 | 10.762 | * | 0.000 | 0.000 | 0.142 | 0.012 | * | |||
| 5-Pregnan-17-ol-3,20-dione | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.176 | 0.007 | * | 3.027 | 0.343 | * | ||||
| 5a-Androstan-3,17-dione | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||
| LNCaP | 22Rv1 | LNCaP | 22Rv1 | LNCaP | 22Rv1 | LNCaP | 22Rv1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AKR1C1 | p | AKR1C3 | p | SRD5A1 | p | HSD3B2 | p | |||||
| Vehicle | 1 | 60.08 | * | 1 | 49.04 | * | 1 | 0.78 | 1 | 10.06 | * | |
| 22-OH-cholesterol | 1 | 40.88 | * | 1 | 38.98 | * | 1 | 0.69 | 1 | 9.40 | * | |
| Pregnenolone | 1 | 59.08 | * | 1 | 66.63 | * | 1 | 0.73 | 1 | 8.77 | * | |
| Progesterone | 1 | 18.82 | * | 1 | 69.94 | * | 1 | 0.37 | * | 1 | 10.09 | * |
| 17-OH-pregnenolone | 1 | 41.08 | * | 1 | 62.20 | * | 1 | 0.54 | 1 | 6.77 | * | |
| 17-OH-progesterone | 1 | 18.12 | * | 1 | 40.56 | * | 1 | 0.38 | * | 1 | 8.33 | * |
| LNCaP | 22Rv1 | LNCaP | 22Rv1 | LNCaP | 22Rv1 | LNCaP | 22Rv1 | |||||
| HSD17B3 | p | HSD17B6 | p | RDH5 | p | STAR | p | |||||
| Vehicle | 1 | 11.61 | * | 1 | 9.05 | * | 1 | 31.34 | * | 1 | 4.70 | * |
| 22-OH-cholesterol | 1 | 11.66 | * | 1 | 6.11 | * | 1 | 22.31 | * | 1 | 4.52 | * |
| Pregnenolone | 1 | 22.98 | * | 1 | 4.05 | * | 1 | 40.44 | * | 1 | 7.60 | * |
| Progesterone | 1 | 22.53 | * | 1 | 8.07 | * | 1 | 35.02 | * | 1 | 7.95 | * |
| 17-OH-pregnenolone | 1 | 14.36 | * | 1 | 2.81 | * | 1 | 20.28 | * | 1 | 5.55 | * |
| 17-OH-progesterone | 1 | 23.08 | * | 1 | 4.48 | * | 1 | 33.27 | * | 1 | 7.95 | * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, S.; Pham, S.; Ming, D.-S.; Chin, M.Y.; Adomat, H.; Hurtado-Coll, A.; Gleave, M.E.; Guns, E.S.T. Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models. Cancers 2018, 10, 343. https://doi.org/10.3390/cancers10100343
Deb S, Pham S, Ming D-S, Chin MY, Adomat H, Hurtado-Coll A, Gleave ME, Guns EST. Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models. Cancers. 2018; 10(10):343. https://doi.org/10.3390/cancers10100343
Chicago/Turabian StyleDeb, Subrata, Steven Pham, Dong-Sheng Ming, Mei Yieng Chin, Hans Adomat, Antonio Hurtado-Coll, Martin E. Gleave, and Emma S. Tomlinson Guns. 2018. "Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models" Cancers 10, no. 10: 343. https://doi.org/10.3390/cancers10100343






