Abstract
Photocatalytic conversion of CO2 to useful products is an alluring approach for acquiring the two-fold benefits of normalizing excess atmospheric CO2 levels and the production of solar chemicals/fuels. Therefore, photocatalytic materials are continuously being developed with enhanced performance in accordance with their respective domains. In recent years, nanostructured photocatalysts such as one dimensional (1-D), two dimensional (2-D) and three dimensional (3-D)/hierarchical have been a subject of great importance because of their explicit advantages over 0-D photocatalysts, including high surface areas, effective charge separation, directional charge transport, and light trapping/scattering effects. Furthermore, the strategy of doping (metals and non-metals), as well as coupling with a secondary material (noble metals, another semiconductor material, graphene, etc.), of nanostructured photocatalysts has resulted in an amplified photocatalytic performance. In the present review article, various titanium dioxide (TiO2)-based nanostructured photocatalysts are briefly overviewed with respect to their application in photocatalytic CO2 conversion to value-added chemicals. This review primarily focuses on the latest developments in TiO2-based nanostructures, specifically 1-D (TiO2 nanotubes, nanorods, nanowires, nanobelts etc.) and 2-D (TiO2 nanosheets, nanolayers), and the reaction conditions and analysis of key parameters and their role in the up-grading and augmentation of photocatalytic performance. Moreover, TiO2-based 3-D and/or hierarchical nanostructures for CO2 conversions are also briefly scrutinized, as they exhibit excellent performance based on the special nanostructure framework, and can be an exemplary photocatalyst architecture demonstrating an admirable performance in the near future.
1. Introduction
Enormous amounts of CO2 emissions, mainly due to industrialization and burning of fossil fuels, are considered to be a primary source of global warming [1]. Hence, fossil fuel consumption for fulfilling energy demands has led to increased atmospheric CO2 levels, along with depletion of respective resources. To deal with such a critical energy and environmental issue, developments in the field of renewable energy such as wind, hydel, biomass, nuclear and solar energy, are being carried out by global scientists and researchers. Among these, solar energy, in terms of its utilization in converting anthropogenic CO2 into value-added chemicals on a photocatalyst surface in the presence of a reducing agent, is an alluring and auspicious research area to counter environmental pollution with the possibility of matching the renewable energy infrastructure [2]. The photocatalytic CO2 conversion (PCC) to value-added chemicals like CO, CH4, C2H6, C2H5OH, C2H4, CH3OH, HCOOH, etc., in general mimics the concept of natural photosynthesis and is considered a subordinate of the “Artificial Photosynthesis” research domain [2,3,4,5].
Since the invention of water photocatalysis by Fujishima and Honda in 1972 [6], TiO2 photocatalysts have emerged as the premier and champion material with splendid properties including favorable surface area, non-toxicity, abundant availability, high stability, and cost effectiveness [7,8,9]. On the contrary, TiO2 nanoparticles (0-D), with a disadvantage of only UV light absorption, also exhibit drawbacks of fast electron–hole recombination, slow charge transfer, limited light trapping and difficulty in reuse/recycling [10]. Despite the development of many alternative photocatalysts to TiO2, TiO2 still remains a distinguished and premier choice because of the specific above-mentioned attributes.
In the last few years, TiO2-based nanostructured photocatalysts such as one-dimensional (1-D) [11,12,13], two-dimensional (2-D) [14,15,16], and three-dimensional (3-D) or hierarchical structures [17,18], have received massive attention in a variety of photocatalysis domains [19]. The distinct properties of large surface area, high aspect ratio, directional flow of photogenerated charges resulting in decreased charge recombination, light scattering, stability and improved reusability/recyclability—especially for 1-D arrays and hierarchical nanostructures—has made them valuable and worthwhile to employ in the photocatalysis research domain. Moreover, the synthesis strategies for the 1-D nanostructures are simple and facile; however, for 2-D and hierarchical nanostructured photocatalysts, fabrication is generally limited to following/adopting complicated procedures. Hence, due to their specific geometry configurations [20,21], 1-D (nanotubes, nanowires, nanorods, etc.) and 2-D (nanosheets, nanolayers, etc.) nanostructured photocatalysts provide an attractive and exemplary opportunity to overcome the limitations of 0-D nanoparticles restricting the performance of photocatalysts. Furthermore, 3-D and hierarchical nanostructures offer superb light trapping properties within specific nanostructures, resulting in a slow photon effect and improved photocatalytic performance. Despite the eminent benefits of TiO2 nanostructured photocatalysts, to some extent, they possess limitations with respect to the ultra violet region (a small portion of terrestrial solar spectrum) in terms of light absorption due to their wider band gap (~3.0–3.2 eV). Therefore, to overcome such limitations, similar strategies are commonly adopted as for 0-D nanoparticles, including metal and non-metal doping [18,22,23,24,25], noble metal loading [16,26,27,28,29], graphene derivative coupling [30,31,32,33], and hetero-junctioning TiO2 nanostructures through the coupling of low band gap materials [11,17,34,35,36,37].
In this review, the recent progress of TiO2-based nanostructures employed for photocatalytic CO2 conversion to useful/value added chemicals is briefly overviewed. The key parameters promoting the improvement of photocatalytic performance, as well as the potential benefits offered by important frameworks, i.e., 1-D, 2-D and hierarchical nanostructures, are discussed. The photocatalytic performance of the TiO2-based nanostructured photocatalysts, along with their reaction conditions and procedures, is summarized. In short, this review is focused on the architectural engineering of TiO2-based nanostructured photocatalysts, such that they offer excellent properties with enhanced photocatalytic performance.
2. Photocatalytic CO2 Conversion: Fundamentals and Mechanism
As is well established, CO2 in gaseous form is a thermodynamically stable molecule with a Gibbs free energy of ΔG° = −394.4 KJ∙mole−1 [38]. Thus, a suitable amount of energy is required to transform gaseous CO2 into value-added products. Equation (1) (Gibbs free energy) and Equation (2) (overpotential) demonstrate that more negative potential than E° is needed to compensate the overpotential and make the ΔG° negative enough for spontaneous conversion of CO2 gaseous molecule to proceed.
where ΔG° = Standard Gibbs free energy, n= number of electrons involved in reaction, F = Faraday constant (96485 Cmole−1) and E° = standard potential of the respective reaction.
where η = overpotential, E = required potential and E° = standard potential.
ΔG° = nFE°
η= E − E°
Hence, the required overpotential can be supplied to the reaction mixture in one of several ways, including as thermal energy, electrical energy, chemical energy and solar energy. Among these sources, utilization of solar energy in the presence of a photocatalyst is considered to be one of the most sustainable and cost-effective approaches. Moreover, for the reason of high stability, it is quite difficult for a photocatalyst alone to reduce CO2 into products; therefore, the reduction to CO2 proceeds with the support of reducing agents such as H2O, H2, etc. [38,39], which can regenerate the photocatalyst by readily providing the electrons in order to fill the holes. In turn, protons are released, which react with the surface-adsorbed CO2 and electrons to give the desired product: a relatively feasible and less energetic pathway. Such a process is commonly known as a “proton-assisted multi-electron photoreduction process”, and it is a commonly accepted mechanism for photocatalytic CO2 conversion. Table 1 shows the redox potentials for various CO2 reactions (at pH = 7.0 and E vs. NHE) with H2O in vapor or liquid phase [40]. It can be noted that the selectivity of the product, another important parameter, can also be manipulated by aligning the conduction band and valence band edge of the photocatalyst with respect to the redox potentials of the reactions.

Table 1.
Electrochemical redox potentials (E° vs. NHE, pH = 7.0) for CO2 reduction into a variety of useful chemical products [4,40,41].
As mentioned above, the conversion or reduction of CO2 is more feasible in the presence of reducing agents such as H2O, H2, etc. H2O is commonly chosen as a reducing agent because it has benefits such as being inexpensive, less dangerous than gaseous reducing agents (H2, H2S, etc.), and it only requires simple handling. Thus, a reaction mixture of gaseous CO2 and H2O (vapors or liquid) or H2 gas is commonly used for the photocatalytic conversion of CO2 into the desired products. Upon light irradiation on the photocatalyst with a CO2(g)/H2O(g/l) or CO2(g)/H2(g) mixture, the photogenerated electrons are rapidly transferred to the adsorbed CO2 on the photocatalyst surface, and the presence of protons (H+, which are provided by the reducing agents) yields the desired products. The definitive reaction mechanism for photocatalytic CO2 conversion is still in need of extensive research; however, based on the binding and bridging mode of CO2 with a photocatalyst surface, two commonly accepted pathways reported in the literature [42,43,44,45] include (i) the carbene pathway, and (ii) the formaldehyde pathway.
The possible reactions reported in the literature for the carbene pathway are presented in Equations (3)–(11). It is expected that CO2●− radicals are formed by the quick reaction of a single electron to the surface-adsorbed CO2. These radicals can then react with protons/hydrogen radicals and electrons to form CO as an intermediate product, which is believed to be adsorbed onto the photocatalyst surface, further reacting with electrons and protons in a multi-step process to produce CH● radical, carbene, methyl radical and finally methanol or methane as valuable products.
CO2 + e− → CO2●−
CO2●− + e− + H+ → CO + OH−
CO + e− → ●CO−
●CO + e− + H+ → C + OH−
C + e− + H+ → CH●
CH● + e− + H+ → CH2
CH2 + e− + H+ → CH3●
CH3● + e− + H+ → CH4
CH3 + OH− → CH3OH
In the formaldehyde pathway, the monodentate configuration via binding of one oxygen atom to a metal (titanium) atom, or binding of materials surface oxygen atom to the carbon atom of CO2, favors the formation of carboxyl radical (COOH). Carboxyl radical (COOH) can react with the protons/hydrogen radicals, forming formic acid, which proceeds through the multi-step process of electron accepting and dehydration reactions to produce formaldehyde, methanol and methane. The proposed reactions are presented in Equations (12)–(21).
CO2 + e− → CO2●−
CO2●− + H+ → ●COOH
●COOH + e− + H+ → HCOOH
HCOOH + e− + H+ → H2OOC●
HC●OOH + e− + H+ → HCOH + H2O
HCOH + e− → H2C●O−
H2C●O− + H+ → H2OHC●
H2OHC●+ e− + H+ → CH3OH
CH3OH + e− + H+ → ●CH3 + H2O
●CH3 + e− + H+→ CH4
3. One-Dimensional (1-D) Nanostructured Photocatalysts
One dimensional (1-D) TiO2 nanostructures, such as nanotubes, nanowires, and nanorods, have been a topic of great interest within the photocatalysis research domain, and they have a variety of applications. The key advantages offered by 1-D TiO2 nanostructures include large surface areas, better charge transfer, improved adsorption capacity, and extended light absorption due to the light trapping and scattering effect [46]. Until now, a moderate amount of research has been done on the development of one-dimensional (1-D) TiO2 photocatalysts with a key focus being on photocatalytic CO2 conversion to hydrocarbon fuel/useful chemicals. Several strategies encompass heterojunction formation with other visible light active photocatalysts, doping, noble metals loading and graphene coupling for attaining the key aim of improved photocatalytic CO2 conversion.
Xin et al. proposed CdS and Bi2S3 heterostructured TiO2 nanotubes (CdS-TNT and Bi2S3-TNT) photocatalysts prepared by a simple two-step synthesis approach [47]. TNT was first prepared using an alkaline hydrothermal method, followed by deposition of CdS and Bi2S3 in a fixed concentration using a simple precipitation approach from their respective precursors. The prepared photocatalysts were tested under visible light irradiation for photocatalytic CO2 conversion. The Bi2S3-TNT photocatalyst showed the maximum efficiency, yielding 224.6 µmol/L of CH3OH after 5 h of irradiation, as compared to CdS-TNT (159.5 µmol/L) and pure TNT (102.59 µmol/L). The increased production rate is mainly attributed to the extended light absorption of heterostructured photocatalysts and their efficient photogenerated charge separation. The conduction band edges of both CdS and Bi2S3 lie well above the conduction band edge of TiO2; therefore, upon light irradiation, the excited electron can easily flow to the conduction band of TiO2 and might react with the adsorbed CO2 species. A depiction of the band gap alignment and CO2 conversion to CH3OH is displayed in Figure 1a. Moreover, it was found that CdS and Bi2S3 deposition decreased the surface area and CO2 adsorption, in comparison to pure TNT, but did not significantly affect the photocatalytic performance.
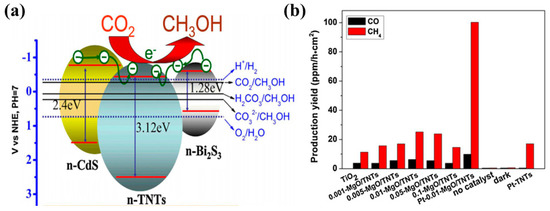
Figure 1.
(a) Proposed mechanism of photocatalytic CO2 conversion to CH3OH employing CdS and Bi2S3 TNT photocatalysts (taken with permission from [47]). (b) Production yield of CO and CH4 from Pt-MgO-covered TNN films (taken with permission from [48]).
Another effective approach is coating the 1-D photocatalyst surface with a good CO2 adsorbent, such as MgO-covered TiO2 nanotube networks (TNN) [48]. The TNN were fabricated by subjecting Ti foil to alkaline hydrothermal reaction, which resulted in well-aligned TNN. The TNN was then dipped in magnesium salt solutions of various concentrations, followed by calcination in air to finally obtain MgO-covered TNN. It was observed that MgO coverage with optimum concentration displays higher CO and CH4 yields when compared with bare TNN. Moreover, the deposition of platinum nanoparticles (Pt NPs) onto MgO-TNN greatly enhances the CO and CH4 yield. The increased performance of MgO-TNN is attributed to the chemisorption of CO2 molecules and their conversion to MgCO3 species, which are more reactive with atomic H than linear CO2 molecules in terms of giving the respective products. In addition, Pt NPs act as an electron trap for the efficient separation of photogenerated electron–hole pairs. The photocatalytic conversion of CO2 into CO and CH4 when employing various MgO-TNN and Pt-MgO-TNN films is displayed in Figure 1b.
Self-doping, such as the introduction of oxygen vacancies into the TiO2, generally referred to as reduced TiO2, is another attractive strategy for improving the photocatalytic performance. A recently published article reported the synthesis of black TiO2 films comprised of unique porous grid-like structures using a simple and safe hydrothermal method [49]. The elemental analysis of the synthesized TiO2 films displayed oxygen deficiency, indicating the presence of oxygen vacancies. When employed for photocatalytic CO2 conversion, the black TiO2 films exhibited improved CO and CH4 yields as compared to pure TiO2 film. The increased product yield was mainly attributed to the extended light absorption and the efficient charge separation resulting from the degree of defects caused by the oxygen vacancies. Another study proposed a novel heterostructure consisting of octahedral Cu2O nanoparticle-loaded TiO2 nanotube (TNT) arrays [50]. TNT arrays were prepared by a conventional electrochemical anodization method, while Cu2O nanoparticles were deposited from Cu salt solution using an electrodeposition approach. The Cu2O-TNT arrays were prepared with varied Cu2O deposition times. It was observed that the Cu2O-TNT arrays prepared with an electrodeposition time of 30 min showed the maximum CH4 yield under visible light irradiation. However, under simulated solar light illumination, the Cu2O-TNT arrays prepared with an electrodeposition time of 15 min exhibited the maximum CH4 yield. The key factors to which the enhanced performance was attributed include: (i) TNT arrays provide better charge transportation and light absorption, (ii) optimum loading of Cu2O nanoparticles leading to improved visible light performance, and (iii) well-aligned band edges of Cu2O and TNT arrays leading to better charge separation. The proposed mechanism involved is displayed in Figure 2a.
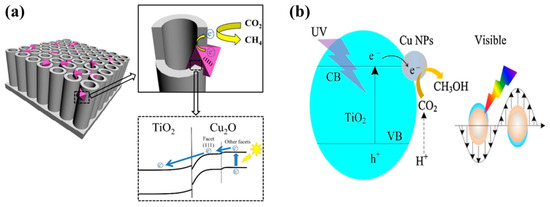
Figure 2.
Schematic of the mechanism involved in the photocatalytic conversion of CO2 into: (a) CH4 employing Cu2O NP-incorporating TNT (taken with permission from [50]), and (b) CH3OH using Cu-modified TNF films (taken with permission from [51]).
Another investigation reported a simple strategy for synthesizing Cu-modified TiO2 nanoflower films (TNF) for enhanced photocatalytic conversion of CO2 into CH3OH [51]. The synthesis methodology includes the growth of TNF on Ti foil using a hydrothermal method, followed by a microwave-assisted reduction process for Cu modification. The Cu-TNF with optimum loading of Cu (0.5 millimol concentration of Cu2+) exhibited enhanced CH3OH yield under UV-visible and UV light irradiation conditions, at a factor of 6 and 3.6 times higher than pure TNF.. The increased CH3OH yield was mainly attributed to good charge separation and local surface Plasmon resonance (LSPR), which were induced by Cu NPs providing hot electrons to contribute to the photocatalytic reactions. The possible mechanism involved in the photocatalytic conversion of CO2 into CH3OH is depicted in Figure 2b.
Recently, Cheng et al. reported CdS and Cu2+ ion deposition onto TiO2 nanorod (TNR) array film, and investigated its performance under visible light irradiation [52]. The TNR film was synthesized using a hydrothermal approach, while Cu2+ ions and CdS deposition were obtained using the cation adsorption and successive ionic layer reaction (SILAR) methods. Various samples were prepared by varying CdS deposition by SILAR cycles. The CdS-Cu2+/TNR film prepared with 2 SILAR cycles showed the maximum ethanol yield under visible light irradiation, thus suggesting this to be the optimum sample. Moreover, the influence of the CO2 flow rate and reaction temperature was also analyzed, and the optimal conditions for yielding maximum ethanol yield were found to be 4 mL/L and 80 °C.
Li et al. recently proposed a heterostructure of TiO2 nanotubes with CoOx, fabricated with a specially designed synthesis strategy for grafting CoOx nanoparticles onto TNT and inducing defects through hydrogenation within the heterostructure by N2/H2 annealing [53]. The TNT employed in the investigation consisted of TiO2 (B) and anatase (A) phases. Two different samples were prepared by selecting the hydrogenation step before and after the deposition of CoOx NPs, denoted AB-H-CoOx and AB-CoOx-H, respectively. Moreover, a new concept of photothermal catalytic conversion (PTC) was employed for conversion of CO2 into useful products. It was observed that the TNT-CoOx sample prepared using the AB-H-CoOx sequence at a temperature of 393 K resulted in a greater CO and CH4 yield as compared to the other samples. The key parameters associated with this performance enhancement includes: (i) improved surface area, (ii) oxygen vacancies, which increase the CO2 adsorption on the surface sites, and (iii) upon light irradiation, the photogenerated electrons are trapped by oxygen defects, whereas the holes are rapidly transported by CoOx clusters, thereby resulting in efficient charge separation. The synthesis methodology and CO2 conversion rates are displayed in Figure 3.
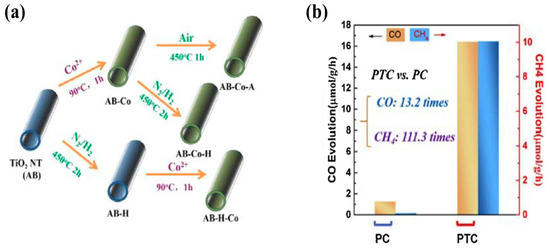
Figure 3.
(a) Synthesis procedure for hydrogenated TiO2 nanotubes CoOx photocatalyst. (b) Photocatalytic CO and CH4 yield employing the AB-H-CoOx sample using photocatalytic and photothermal catalytic approaches (taken with permission from [53]).
Noble metal loading, such as with silver (Ag) and gold (Au) nanoparticles, is also an effective strategy for improving the photocatalytic performance of the material. A report presented a size-controlled study of 1-D TiO2 nanowires (TNW) loaded with Au NPs for photocatalytic conversion CO2 into value-added chemicals [54]. The TNW were synthesized using an alkaline hydrothermal approach followed by deposition of Au NPs using a well-established chemical reduction method. The loading with the Au NPs was optimized by varying the concentration of the respective salts. The 0.5% Au-TNW exhibited the highest yields of CO, CH4 and CH3OH (1237, 13 and 12.6 µmol/g h, respectively) under visible light irradiation as compared to pure TNW (9, 3 and 0 µmol/g h). Moreover, the effect of TNW size was also investigated, and it was found that TNW synthesized with a reaction time of 2 h offered the best performance due to its uniform and smooth surfaces and increased surface areas. Upon loading with Au NPs, the surface area was reduced a bit, but the performance was improved due to the synergetic effect of Au NPs, with smaller-sized NPs acting as electron extractors, while larger NPs injected hot electrons into the TNW conduction band as a result of the LSPR effect. The proposed mechanism for the Au-TNW in photocatalytic CO2 conversion is well displayed in Figure 4a. Similarly, TNW loaded with Ag nanoparticles [55] or a combination of both Au and Ag nanoparticles [56] exhibited an analogous performance attitude under UV and visible light irradiation.. Another similar strategy involves the electrodeposition of Ag nanoparticles on the inner side of TiO2 nanotube (TNT) arrays [57]. The photocatalytic performance of Ag-TNT with respect to the conversion of CO2 was evaluated against pure TNT and Ag-TNT fabricated using a chemical bath deposition approach. The electrodeposited Ag NPs at a fixed voltage and time resulted in a uniform size distribution and good junctioning to the inner side of the TNT arrays. This heterostructure resulted in an enhanced CH4 yield with a small amount of CH3OH under light irradiation, as compared to pure TNT arrays and those of Ag-TNT arrays fabricated using conventional chemical bath deposition. The performance enhancement of the prepared photocatalyst was attributed to the light scattering inside the TNT arrays, which led to a greater degree of light absorption by Ag NPs, thus intensifying the LSPR effect. A more intensified LSPR effect leads to the injection of hot electrons into the conduction band of TNT, and thus to efficient charge separation and reaction with the adsorbed CO2 to yield CH4 or CH3OH. Su et al. proposed the deposition of palladium (Pd) nanoparticles onto TiO2 nanowires (Pd-TNW) and evaluated photocatalytic performance by CO2 conversion to CO and CH4 [58]. TNW were synthesized using a hydrothermal approach, while Pd nanoparticles were deposited using a chemical reduction method. The Pd deposition was optimized by varying the Pd salt concentration, and it was found that 0.5% Pd-TNW exhibited the maximum CO and CH4 yield, which was mainly attributed to the efficient electron separation of Pd from the TNW conduction band, resulting in rapid reaction with the adsorbed CO2.
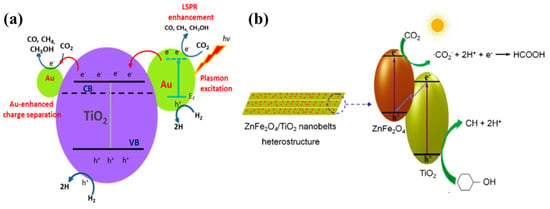
Figure 4.
(a) Schematic presentation of the proposed photocatalytic conversion of CO2 into CO, CH4, CH3OH and other value-added chemicals employing Au-TNW (taken with permission from [54]). (b) Z-scheme proposed for ZnFe2O4-TNB for CO2 conversion to its respective products (taken with permission from [59]).
Another research work proposed the formation of heterostructures with a visible light active photocatalyst: ZnFe2O4 nanoparticles with TiO2 nanobelts (TNB) [59]. The ZnFe2O4 nanoparticles were allowed to grow onto the TNB and tested for photocatalytic conversion of CO2 in cyclohexanol to cyclohexanone (CH) and cyclohexyl formate (CF). With the optimum concentration of ZnFe2O4 nanoparticles, i.e., 9.78%, the CH and CF yields approached their maximum; beyond this concentration, the yields decreased due to the flight shielding effect of heavy loading. The enhanced performance was attributed mainly to the efficient charge separation by a Z-scheme mechanism; Figure 4b.
Another research work proposed replacing the noble metal loading with rGO sheets deposited onto TiO2 nanotube arrays (TNT). Interestingly, using the designed synthesis strategy, the resulting photocatalyst consisted of TNT arrays covered with rGO sheets with embedded TiO2 nanoparticles [30]. When employed for photocatalytic CO2 conversion, the resulting photocatalyst provided a CH4 yield that was 4.4 times higher than that achieved with pure TNT arrays, which was mainly attributed to the improved charge separation and extended light absorption. This proposed scheme for photocatalytic CO2 conversion is displayed in Figure 5a.
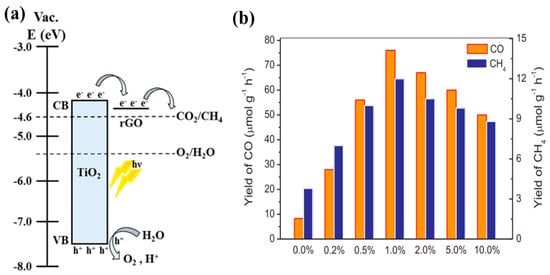
Figure 5.
(a) Schematic view of photocatalytic conversion of CO2 into CH4 on rGO-TNT (taken with permission from [30]). (b) Production rate of CO and CH4 from GR-TNR with varied concentrations of graphene (taken with permission from [60]).
Similarly, various TiO2 nanostructures, i.e., nanoparticles, nanotubes and nanosheets combined with graphene, were have been synthesized and studied for the purposes of photocatalytic conversion of CO2 into useful products [60]. Different synthesis procedures were adopted for each nanostructure; however, TiO2 nanorods (TNR) were synthesized using a hydrothermal method and graphene compositing was also performed using the hydrothermal approach. The 1% graphene-TNR (GR-TNR) exhibited the highest yields of CO and CH4, which was mainly attributed to the increased surface area and the better interaction of graphene and TNR leading to improved charge separation under UV and visible light irradiation. The CO and CH4 yields from the various GR-TNR investigated are shown in Figure 5b.
Recently, Kar et al. proposed a novel strategy using flame-annealed TNT, resulting in mixed-phase (rutile and anatase) square-shaped nanotubes with defect-induced oxygen vacancies [61]. The TiO2 nanotubes were fabricated using a conventional electrochemical anodization method, employing a water base and an ethylene glycol base as an electrolyte. The synthesized TNT were then flame-annealed using a propane torch at a temperature of 750 °C for about 2 min. The flame-annealed TNT (FANT) using a water-based electrolyte exhibited the highest CH4 yield under simulated solar light and visible light irradiation of a 50 W LED lamp, and this was around 1.7 and 1.56 times higher than TNT. The improved performance of FANT (TNT prepared with a water-based electrolyte) was mainly attributed to the improved light absorption achieved by using a mixed phase of FANT, i.e., anatase and rutile, along with the well-aligned band edges of the rutile phase, which were 0.2 eV lower than in the anatase phase, resulting in efficient charge separation. The presence of Ti3+ states was also observed, which might have been generated due to improper oxidation of TNT; hence, extending light absorption as a result of the presence of shallow defects. Moreover, the most important aspect of the square shape of FANT is its interaction with light, resulting in a higher density of electromagnetic hotspots, and thus also contributing to improved photocatalytic performance. The synthesis procedure for FANT and the proposed mechanism involved in photocatalytic CO2 conversion are shown in Figure 6.
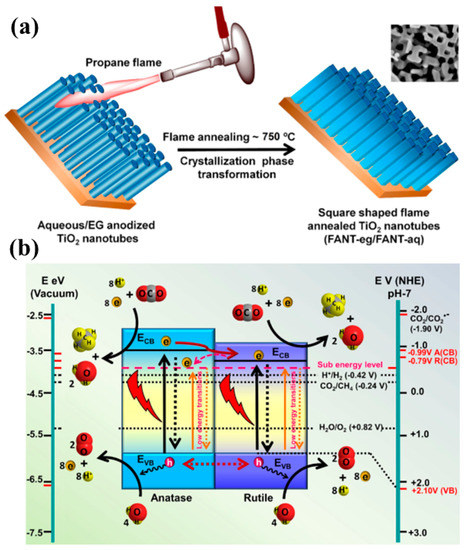
Figure 6.
(a) Schematic overview of the synthesis procedure for FANT, and (b) the proposed mechanism for photocatalytic conversion of CO2 into CH4 employing the FANT photocatalyst (taken with permission from [61]).
A summarized overview of the numerous TiO2-based 1-D nanostructures reviewed, including product type and production rate, reaction conditions, and key process parameters influencing photocatalytic performance, is presented in Table 2.

Table 2.
Summary of various 1-D nanostructured photocatalysts, including reaction conditions, production of value-added chemicals by photocatalytic CO2 conversion, and key parameters for performance improvement.
4. Two Dimensional (2-D) Nanostructured Photocatalysts
Fabrication of two-dimensional (2-D) nanostructured photocatalysts is another interesting and effective strategy for achieving improved photocatalytic activity. The relevant research domains have been widely investigated due to the intriguing properties they offer, which include large surface area, improved surface adsorption, improved interfacial charge transfer, and band edge alignment leading to product selectivity. With regard to photocatalytic CO2 conversion, a moderate amount of work has been done on the development of TiO2-based 2-D nanostructured photocatalysts with improved performance resulting from combinations of the factors mentioned above.
Loading TiO2 nanoparticles onto graphitic carbon nitride (g-C3N4) layered nanosheets was investigated by Zhou et al. for the purposes of photocatalytic CO2 conversion [62]. Various photocatalysts were prepared with varied amount of urea as a precursor of g-C3N4. Interestingly, it was observed that when the amount urea was lower than a certain limit, it acted as an N-doping source of TiO2, leading to the formation of N-doped TiO2; however, upon increasing the urea concentration, a composite of g-C3N4 and N-TiO2 was obtained. Such variation resulted in the selectivity of the product, yielding CH4 for N-TiO2 and CO for the g-C3N4/N-TiO2 composite. The product selectivity is attributed to the alignment of the band edges with respect to the redox potentials of prospective products. The g-C3N4/N-TiO2 with 70:30 mole ratio resulted in the maximum yield of CO only, and no CH4. However, the sample prepared with 50:50 mole ratio exhibited production of both CO and CH4. The increased photocatalytic yield of CO and CH4 was mainly attributed to the visible light absorption, moderate surface areas, enhanced interfacial charge transfer, and the alignment of band edges in accordance with product redox potentials. The transmission electron microscopy (TEM) image of the layered structure of N-TiO2 nanoparticles loaded onto g-C3N4 nanosheets is displayed in Figure 7a. The proposed mechanism for the g-C3N4/N-TiO2 is shown in Figure 7b.
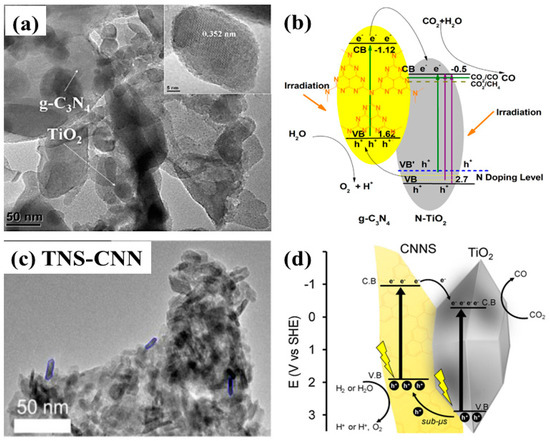
Figure 7.
(a) TEM image showing the loading of N-TiO2 nanoparticles onto g-C3N4 nanosheets, and (b) proposed scheme involved in photocatalytic CO2 conversion (taken with permission from [62]). (c) TEM image showing the 2-D nanostructure of TiO2 nanosheets coupled with g-C3N4 nanosheets, and (d) schematic view of the interfacial charge transfer within TNS-CNN with the proposed photocatalytic CO2 conversion mechanism (taken with permission from [63]).
Similarly, another recently published report proposed an in situ pyrolysis approach for the synthesis of a TiO2 nanosheets-g-C3N4 nanosheets nanostructure (TNS-CNN) [63]. Figure 7c shows the TEM image for the TNS-CNN, clearly displaying the fractions of TNS on the CNN. TNS-CNN has been employed for photocatalytic CO2 conversion using both H2O and H2 as reducing agents. It was observed that TNS-CNN provided a CO yield almost 12 times higher than pristine TNS and 37% higher than TiO2-P25 (with H2 as a reducing agent). When H2O was employed as a reducing agent, the CO yield decreased to one third that obtained when using H2 as a reducing agent. This is mainly attributed to the competition for H2 produced as a side reaction of water splitting, along with its reducing capability. The overall performance might be attributed to the increased surface area, the enhanced interfacial charge transfer between the nanosheets, the effectiveness of H2 as a reducing agent, and the visible light absorption of the CNN. A schematic representation of the CO2 conversion mechanism is shown in Figure 7d.
Recently, Shi et al. reported defect-rich TiO2 quantum dots (QDs) embedded within g-C3N4 nanosheets (TiO2−x/g-C3N4) for efficient photocatalytic conversion of CO2 into CO under solar light irradiation [64]. The fabricated TiO2−x/g-C3N4 nanostructure exhibited a superior photocatalytic performance with a CO yield 5 times higher than that of pristine g-C3N4. The TiO2−x/g-C3N4 was synthesized by a novel and facile strategy of in situ pyrolysis of melamine with MIL-125-NH2 (Ti). Various samples were prepared with varying the mass ratios. The improved photocatalytic performance was mainly attributed to the improved light absorption, which extended towards the red region, the enhanced CO2 adsorption due to the defective TiO2 QDs, the efficient charge separation, as confirmed by transient photocurrent and photoluminescence spectroscopy, and the large surface area due to the nanostructured architecture of the material. Figure 8a displays the SEM image of a representative sample of sheet-type g-C3N4 embedded with 0-D TiO2−x nanoparticles. The proposed photocatalytic mechanism is depicted in Figure 8b, which provides a clear demonstration of the alignment of the band edges to the redox potentials. It can be seen that upon light irradiation TiO2−x/g-C3N4 generates electron–hole pairs, and the electrons from the conduction band of g-C3N4 flow towards the conduction band of TiO2−x, where in the presence of Co(bpy)32+, the co-catalyst reacts sharply with the adsorbed CO2 to provide CO. In contrast, the holes within the TiO2−x and g-C3N4 are regenerated by the Triethanolamine (TEOA) hole scavenger.
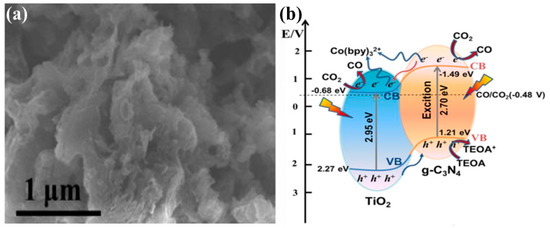
Figure 8.
(a) SEM image of a representative TiO2−x/g-C3N4 sample displaying sheet-type g-C3N4 embedded with TiO2−x nanoparticles, and (b) schematic of the electronic structure of TiO2−x/g-C3N4 with the proposed mechanism of photocatalytic CO2 conversion (taken with permission from [64]).
Ultrathin TiO2 nanosheets, prepared from a TiO2-Octylamine lamella structure, resulted in an efficient photocatalyst for CO2 conversion [65]. The extravagant increase in the surface area of the TiO2 nanosheets led to greater light absorption and an increased number of CO2 adsorption active sites. It was observed that, in order to obtain ultrathin nanosheets, decreasing the bulk thickness towards atomic-scale thickness might provide the surface atoms with efficient surface active sites for photocatalytic CO2 conversion. Moreover, the fluorescence lifetime of the photogenerated charge within the ultrathin TiO2 nanosheets was observed to be higher when compared to their bulk counterparts; therefore, suggesting that the ultrathin nanosheets provide an efficient charge separation pathway along its 2-D channels. As a result of the contributions of such parameters, the formate formation from ultrathin TiO2 nanosheets was around 450 times higher than that of bulk TiO2. Figure 9a shows a SEM image of ultrathin TiO2 nanosheets, while Figure 9b displays a schematic view of the CO2 conversion mechanism.
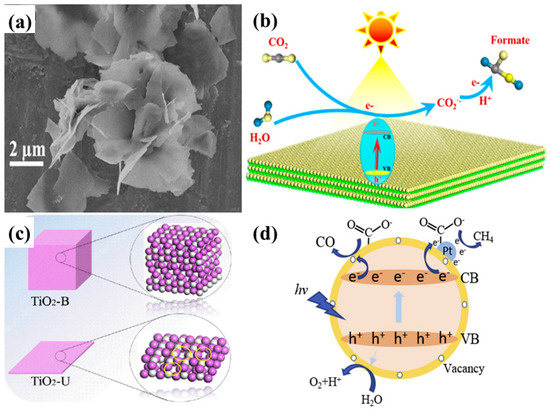
Figure 9.
(a) SEM image of the TiO2-Octylamine hybrid nanostructure, and (b) schematic view of photocatalytic conversion of CO2 into formate product using ultrathin TiO2 nanosheets (taken with permission from [65]). Schematic view of (c) unsaturation of ultrathin nanosheets with the appearance of oxygen vacancies, and (d) photocatalytic CO2 conversion to CH4 with water vapors employing ultrathin TiO2 nanosheets with highly dispersed Pt nanoparticles (taken with permission from [66]).
Recently, Liu et al. reported the fabrication of TiO2 ultrathin nanosheets (TiO2-U) using a simple hydrothermal approach followed by Pt nanoparticle deposition using a photochemical deposition method [66]. The Pt-TiO2-U, when employed for photocatalytic CO2 conversion, resulted in increased CO (54.2 µmol/g h) and CH4 (66.4 µmol/g h) yields when compared to the pristine and reference samples. It was observed that TiO2-U exhibited visible light absorption, indicating the presence of oxygen vacancies, which were confirmed by X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) analysis. The appearance of such self-defects (oxygen vacancies or Ti3+ states) was attributed to the narrowing of the thickness, leading to the formation of uncoordinated surface sites, as shown in Figure 9c. Such defects with respect to Ti3+ promote the adsorption of CO2 and the uniform deposition of Pt nanoparticles. The improved photocatalytic performance was mainly attributed to the increased surface area, extended light absorption and CO2 adsorption sites, and enhanced electron–hole separation by Pt nanoparticles. A schematic view of the photocatalytic mechanism is shown in Figure 9d.
Surface modification of the photocatalyst is another effective approach, leading to improved surface characteristics for photocatalytic CO2 conversion [67]. One research work reported that the acidification of TiO2 nanosheets using sulfuric acid led to enhanced CH4 yield after 4 h of light irradiation. Acidification did not affect the sheet structure or the exposed facet of the material. However, the surface area decreased a bit due to acid molecules plugging the pores. Moreover, the acid treatment extended the light absorption of the material towards the red region; this was mainly ascribed to the generation of oxygen vacancies during the process. Such vacancy formation also enhanced the electron–hole separation under light irradiation, leading to improved photocatalytic performance.
Lamellar structures also promote the photocatalytic activity by adsorbing photoactive species within the layers. Such layered structures with improved surface area and loading with visible active materials can lead to efficient photocatalysts, as proposed by Junior et al. [68]. The authors reported a TiO2 pillared K2Ti4O9 2-D structure loaded with visible light-active Cu2O nanoparticles. The introduction of TiO2 pillars into layered K2Ti4O9 drastically increased the surface area, which was further increased after loading with Cu2O nanoparticles. Also, the loading with Cu2O nanoparticles shifted the light absorption toward the red region, along with efficient charge separation at the interface of TiO2, K2Ti4O9 and Cu2O. All the mentioned parameters improved the photocatalytic yield of CH3OH from moist CO2, with a yield 2 times as high as that obtained from the pristine sample. The schematic depiction of the mechanism involved is presented in Figure 10a.
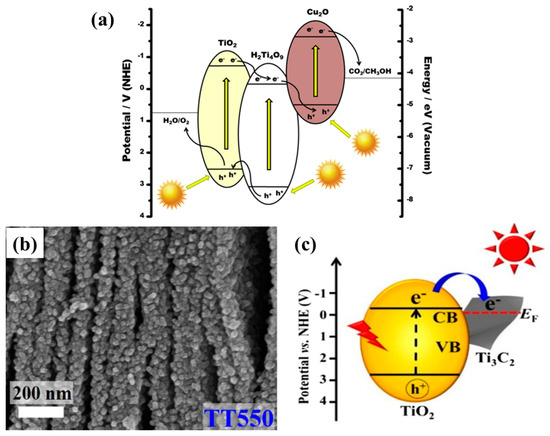
Figure 10.
(a) Schematic view of the band energy diagram for Cu2O-loaded TiO2 pillared photocatalysts for conversion of CO2 into value-added chemicals (taken with permission from [68]). (b) FESEM image for TT550 sample displaying the layered structure of MXenes loaded with TiO2 nanoparticles, and (c) the proposed mechanism of photogenerated charge transfer within the TiO2-Ti3C2 2-D nanostructure (taken with permission from [69]).
Another interesting and excellent approach to developing 2-D nanostructures is the growth of photocatalytic material onto a 2-D conductive substrate. Recently, Low et al. reported a simple approach for synthesizing TiO2 nanoparticles coated onto a conductive Ti3C2 MXenes (TT) [69]. The TiO2 nanoparticles appeared as the Ti3C2 MXenes were calcined at different temperatures and tested for photocatalytic CO2 conversion. When employed, the photocatalytic yield of CH4 was found to be 3.7 times higher than that of commercial TiO2-P25. Figure 10b displays the SEM image of the TT sample calcined at 550 °C, which was the optimum sample. It can be seen that, upon calcination, TiO2 nanoparticles appear on the surface and edges of the layered Ti3C2 MXenes, resulting in a rougher surface and an improved surface area. Upon testing for photocatalytic CO2 conversion, the optimum sample, TT550, yielded the maximum CH4 production, with minor production of CH3OH and C2H5OH. Increasing the temperature beyond this point, the photocatalytic performance decreased due to the lower content of conductive Ti3C2 MXenes. Therefore, it can be concluded that the presence of Ti3C2 MXenes as a conductive pathway offered efficient separation of electron–hole pairs, leading to improved performance. In addition, the improved surface area contributed significantly by providing more reactive sites for CO2 adsorption and conversion. The proposed scheme for photocatalytic CO2 conversion is displayed in Figure 10c.
Another study reported Bi2WO6-TiO2 binanosheets (BT) as a 2-D nanostructure for photocatalytic conversion of CO2 into CO and CH4 [70]. The study mainly aimed to provide an enlightened view regarding carbonaceous intermediates or surface species in the generation of value-added chemicals. However, when tested under CO2 gas, it was observed that the BT sample exhibited enhanced yields of CO and CH4 when compared to the pristine samples. The key causes were ascribed to the improved interfacial charge transfer and the Z-scheme mechanism, which were deemed to be solely responsible for the enhanced photocatalytic performance. Table 3 provides a summarized overview of the numerous TiO2-based 2-D nanostructures employed for photocatalytic conversion of CO2 into various products, with their production rate, reaction conditions and the key process parameters promoting photocatalytic performance.

Table 3.
Summary of various 2-D nanostructured photocatalysts with reaction conditions, value-added chemicals produced as a result of photocatalytic CO2 conversion, and key parameters for improved performance.
5. Hierarchical Nanostructures: A Dynamic and Potent Approach
In recent years, hierarchical nanostructures have received wider attention for the purpose of heterogeneous photocatalysis, as they possess admirable and exemplary properties at the micro/nanometer scale. Since the invention of 3-D/hierarchical nanostructures, intensive research has been carried out with the aim of developing competent and efficient hierarchical nanostructures for a variety of photocatalysis applications [21,72]. Based on nano-sized building blocks such as nanotubes, nanorods, nanosheets, etc., these nanostructures possess superb properties, including porous and interconnected networks, high surface areas, multi-dimensional domains for efficient charge transfer, improved light harvesting, and increased reactant adsorption, which together lead to enhanced photocatalytic performance. Therefore, these captivating aspects of hierarchical nanostructures provide a potentially dynamic opportunity to pursue the development of nanostructured photocatalysts to be employed for the photocatalytic conversion of CO2 into useful chemicals/fuels.
Recently, Wang et al. proposed a 3-D hierarchical TiO2 microsphere (MS) with tunable pore and chamber size to facilitate the diffusion of the gas and its subsequent photocatalytic conversion under simulated solar light irradiation [73]. Three different types of TiO2 microspheres were prepared, i.e., solid MS, yolk/shell MS and hollow MS. Photocatalytic activities were tested under both UV and simulated solar light irradiation. It was observed that under UV light, solid MS exhibted the maximum CO yield (17.7 µmol/g h), which was 1.2 and 1.6 times higher than that obtained with yolk/shell MS and hollow MS, respectively. This increase in yield was attributed to the intense light absorption of solid MS. However, under simulated solar light irradiation, hollow MS showed the maximum CO yield (34 µmol/g, after 3 h of irradiation), which was 1.6 and 1.4 times higher than the yields obtained with solid and yolk/shell MS, respectively. The increased photocatalytic performance under simulated solar light was ascribed to the increased pore size of the hollow MS, leading to rapid diffusion of CO2 molecules towards active sites, which is a result of its unique hierarchical nanostructure. Figure 11 shows SEM images of all of the MS samples and their CO production rate under simulated solar light irradiation.
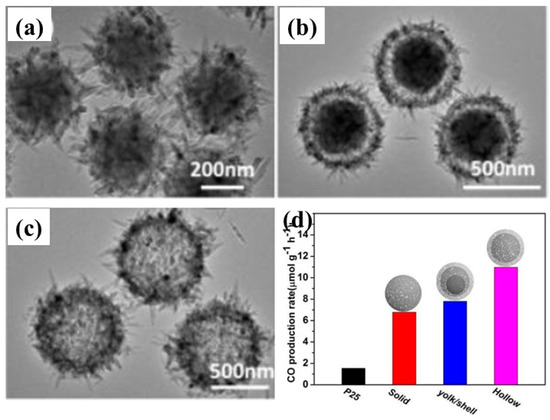
Figure 11.
SEM images of (a) solid MS, (b) yolk/shell MS, and (c) hollow MS. (d) CO production rate for various samples via photocatalytic CO2 conversion (taken with permission from [73]).
Similarly, a recent report presented the replication of Camella tree leaves for synthesis of a unique porous TiO2 architecture with enhanced photocatalytic conversion of CO2 into CH4 and CO [74]. The TiO2 artificial leaves (AL) were synthesized using a bio-template approach. The unique architecture of the AL consists of interconnected nanosheets, leading to improved porosity and a surface area greater than that of TiO2-P25. The photocatalytic CO2 conversion was tested under both UV and visible light irradiation. Under UV light irradiation, the AL yielded mostly CH4, whereas reference nonporous P25 yielded CO. This difference was attributed to the increased residence time and contact between reactant and catalyst within the porous network of the AL. Similar results were obtained when irradiated with visible green light; however, upon loading the Ru2O nanoparticles, the CO and CH4 yields with AL were drastically increased, which can be attributed to the efficient charge separation at the metal–semiconductor junction. Thus, the factors of porous networks with surface defects, increased surface area and efficient charge separation lead to improved photocatalytic performance in the AL hierarchical nanostructure. Figure 12 shows an image of the porous hierarchical TiO2 AL obtained using the bio-template approach, and the rate of photocatalytic conversion of CO2 into CH4 and CO under UV and visible light sources.
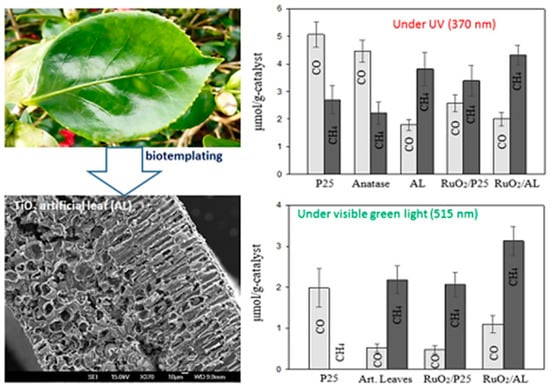
Figure 12.
Image of camellia leaf, SEM image of artificial leaf displaying a porous structure, and the production rate of CO and CH4 via the photocatalytic conversion of CO2 and water vapors (taken with permission from [74]).
Another work presented the fabrication of a 3-D Z-scheme nanostructured photocatalyst consisting of ZnIn2S4 nanosheets assembled onto TiO2 nanobelts [75]. This hierarchical nanostructure, with an optimum ratio of ZnIn2S4 (0.33:1 mole ratio), exhibited an enhanced CH4 yield (1.135 µmol/g h) from the photocatalytic conversion of CO2 and water vapors under UV-visible light irradiation, which is around 39 times higher than the yield obtained from bare ZnIn2S4 nanosheets. The increased CH4 yield was attributed to the improved surface area, light absorption and effective charge separation due to the Z-scheme mechanism. Figure 13a,b shows the CH4 production rate from photocatalytic CO2 conversion.
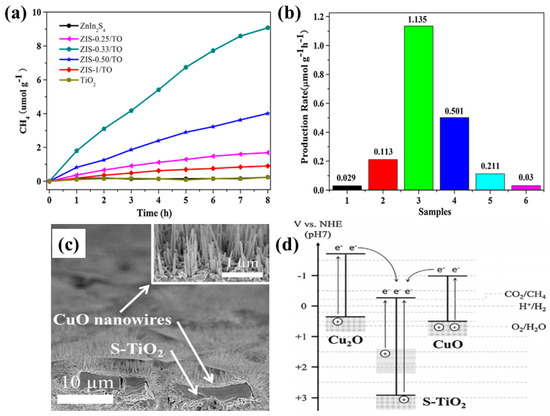
Figure 13.
(a) Photocatalytic CH4 evolution and (b) production rate employing various samples with varied ratios of ZnIn2S4 to TiO2 (taken with permission from [75]). (c) SEM image of Cu2O/S-TiO2/CuO p-n-p nanostructure, and (d) band gap diagram with the mechanism involved in the conversion of CO2 to CH4 (taken with permission from [76]).
Kim et al. developed a unique architecture composed of Cu2O dendrites covered with S-TiO2 micro-blocks and CuO nanowires, resulting in a p-n-p heterojunction formation [76]. When employed in photocatalytic CO2 conversion, this nanostructure yielded a high CH4 production rate, at 2.31 µmole/m2 h, which is ten times higher than with TiO2 nanotubes. The increased CH4 yield was attributed to the improved light absorption and efficient charge separation at the interfaces of the Cu2O dendrites, CuO nanowires and S-TiO2 micro-blocks. Figure 13b shows a SEM image of the nanostructured photocatalyst, while Figure 13d displays the proposed band gap diagram, depicting the charge transfer mechanisms within the nanostructure.
The development of 3-D ordered macroporous TiO2 (3DOM-TiO2) is also an interesting strategy with the aim of improving photocatalytic CO2 conversion. Furthermore, the loading of noble metals like Au, Ag and Pd onto 3DOM-TiO2 results in an efficient photocatalyst nanostructure for improved conversion of CO2 into useful chemicals. As previously reported, AuPd has an optimum ratio of 3:1 weight percent. This was loaded onto 3DOM-TiO2, resulting in an enhanced CH4 yield of 18.5 µmol/g h, whereas AuPd 1:3 exhibited an increased CO yield, 14 µmol/g h, when employing CO2 and water vapors as reactants under UV visible light irradiation [77]. The key parameters responsible for the improved performance were deemed to be the increased light harvesting due to the slow photon effects of 3DOM-TiO2 and the LSPR effect induced by the noble metals, and the efficient charge extraction at the interface of the noble metals and the semiconductors. Figure 14a represents the proposed mechanism for the photocatalytic conversion of CO2 into CH4 and CO when employing 3DOM-TiO2 hierarchical nanostructures loaded with noble metals. Another study presented 3DOM-TiO2 hierarchical nanostructures loaded with Au nanoparticles as an efficient photocatalyst for the conversion of CO2 and water vapors into CH4 under visible light irradiation [78]. The sample with the optimum Au loading exhibited an increased CH4 yield (23.90 µmol/g h) as compared to the pristine sample (11.39 µmol/g h). This increase was attributed to the increased light harvesting due to the slow photon effect, the LSPR due to the uniformly loaded Au nanoparticles, and the effective charge separation at the interface between the Au nanoparticles and 3DOM-TiO2. A representation of the proposed mechanism for the photocatalytic CO2 conversion is depicted in Figure 14b.
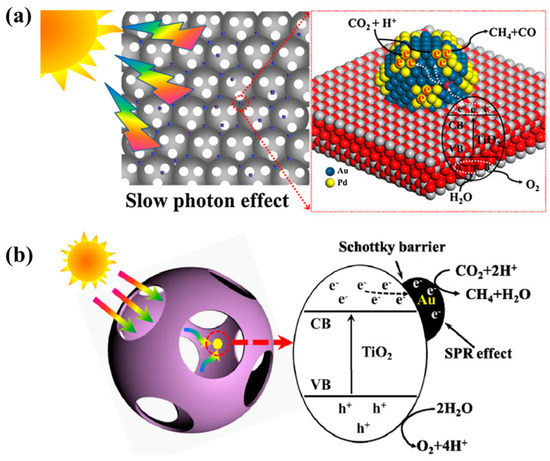
Figure 14.
Proposed mechanism of photocatalytic CO2 conversion with water vapors employing (a) AuPd-3DOM TiO2 photocatalyst (taken with permission from [77]), and (b) Au-3DOM TiO2 photocatalyst (taken with permission from [78]).
6. Conclusions
The present review briefly reveals the influential and imperative aspects of TiO2-based photocatalyst nanostructures, when applied for the purposes of photocatalytic CO2 conversion. The recent developments in TiO2-based nanostructures, i.e., 1-D, 2-D, and hierarchical nanostructures, have made it clear that, based on the geometry and configuration of the nanostructured photocatalyst, improved photocatalytic performance can be ascribed to a combination of (i) large surface areas, (ii) efficient separation of photogenerated charges, (iii) directional charge transport, (iv) improved light harvesting due to light trapping/scattering, and (v) the slow photon effect. Furthermore, the fabrication of hetero-junctioned nanostructured photocatalysts, coupled with noble metals, graphene derivatives, or another semiconductor, multiplies the photocatalytic performance, thus reaping the benefits of both, i.e., the aspects of both nanostructures and hetero-junction formation. Hence, it can be established that the unique and peculiar properties of nanostructured photocatalysts are accompanied by an improvement in photocatalytic performance, and their study is a potent research domain offering excellent prospects for photocatalytic conversion of CO2 into value-added chemicals.
Author Contributions
A.R. collected the data, analyzed it, and wrote the review. S.I. analyzed, modified and rewrote the manuscript.
Funding
The authors gratefully acknowledge the support of the Ministry of Science and ICT (2017R1E1A1A01074890 and 2017M2A2A6A01070912). This research was also supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) funded by the Ministry of Science and ICT (2015M1A2A2074670) as well as by the DGIST R&D Program of the Ministry of Science and ICT (19-BD-0404). Abdul Razzaq would also like to acknowledge the support of Higher Education Commission, Pakistan, under the Start-Up Research Grant for Fresh PhD Holders, Project No. 21-1952/SRGP/R&D/HEC/2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szulejko, J.E.; Kumar, P.; Deep, A.; Kim, K.H. Global warming projections to 2100 using simple CO2 greenhouse gas modeling and comments on CO2 climate sensitivity factor. Atmos. Pollut. Res. 2017, 8, 136–140. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Garcia, H. Solar light photocatalytic CO2 reduction: General considerations and selected bench-mark photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Razzaq, A.; In, S.-I. Development of graphene based photocatalysts for CO2 reduction to C1 chemicals: A brief overview. Catal. Today 2018. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water One and Two-dimensional Structure of Poly (L-Alanine) shown by Specific Heat Measurements at Low. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hoivik, N.; Wang, K.; Jakobsen, H. Engineering TiO2 nanomaterials for CO2 conversion/solar fuels. Sol. Energy Mater. Sol. Cells 2012, 105, 53–68. [Google Scholar] [CrossRef]
- Anpo, M. Photocatalytic reduction of CO2 with H2O on highly dispersed Ti-oxide catalysts as a model of artificial photosynthesis. J. CO2 Util. 2013, 1, 8–17. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, S.; Du, M.; Ye, X.; Wang, Y.; Ye, J. Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysts 2019, 9, 91. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Żebrowska, J.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef]
- In, S.-I.; Nielsen, M.G.; Vesborg, P.C.K.; Hou, Y.; Abrams, B.L.; Henriksen, T.R.; Hansen, O.; Chorkendorff, I. Photocatalytic methane decomposition over vertically aligned transparent TiO2 nanotube arrays. Chem. Commun. Camb. 2011, 47, 2613–2615. [Google Scholar] [CrossRef]
- In, S.-I.; Hou, Y.; Abrams, B.L.; Vesborg, P.C.K.; Chorkendorff, I. Controlled Directional Growth of TiO2 Nanotubes. J. Electrochem. Soc. 2010, 157, E69. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts 2018, 8, 590. [Google Scholar] [CrossRef]
- Qu, J.; Sha, L.; Wu, C.; Zhang, Q. Applications of Mechanochemically Prepared Layered Double Hydroxides as Adsorbents and Catalysts: A Mini-Review. Nanomaterials 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-W.; Verbruggen, S.; Claes, N.; Yadav, A.; Grandjean, D.; Bals, S.; Lievens, P. TiO2 Films Modified with Au Nanoclusters as Self-Cleaning Surfaces under Visible Light. Nanomaterials 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, Z.; He, X.; Yang, X.; Chen, X.; Gao, Z. Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance. Nanomaterials 2019, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts 2017, 7, 344. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Chapter 48—Nanotechnology applications for environmental industry. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Parayil, S.K.; Razzaq, A.; Park, S.M.; Kim, H.R.; Grimes, C.A.; In, S.-I. Photocatalytic conversion of CO2 to hydrocarbon fuel using carbon and nitrogen co-doped sodium titanate nanotubes. Appl. Catal. A General 2015, 498, 205–213. [Google Scholar] [CrossRef]
- Razzaq, A.; Sinhamahapatra, A.; Kang, T.H.; Grimes, C.A.; Yu, J.S.; In, S.-I. Efficient solar light photoreduction of CO2 to hydrocarbon fuels via magnesiothermally reduced TiO2 photocatalyst. Appl. Catal. B Environ. 2017, 215, 28–35. [Google Scholar] [CrossRef]
- Sorcar, S.; Hwang, Y.; Grimes, C.A.; In, S.I. Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO2 reduction into CH4. Mater. Today 2017, 20, 507–515. [Google Scholar] [CrossRef]
- In, S.; Orlov, A.; Berg, R.; García, F.; Pedrosa-Jimenez, S.; Tikhov, M.S.; Wright, D.S.; Lambert, R.M. Effective visible light-activated B-doped and B,N-codoped TiO2 photocatalysts. J. Am. Chem. Soc. 2007, 129, 13790–13791. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.K.; Lim, S.K.; Kim, S.; In, S.I. Efficient visible light-induced H2 production by Au@CdS/TiO2 nanofibers: Synergistic effect of core-shell structured Au@CdS and densely packed TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 166–167, 423–431. [Google Scholar] [CrossRef]
- Lee, H.; In, S.; Horn, M.W. Plasmonic enhancement of CO2 conversion to methane using sculptured copper thin films grown directly on TiO2. Thin Solid Films 2014, 565, 105–110. [Google Scholar] [CrossRef]
- Kim, M.; Razzaq, A.; Kim, Y.K.; Kim, S.; In, S.I. Synthesis and characterization of platinum modified TiO2-embedded carbon nanofibers for solar hydrogen generation. RSC Adv. 2014, 4, 51286–51293. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Saito, N.; Hunsom, M. Photoinduced Glycerol Oxidation over Plasmonic Au and AuM (M = Pt, Pd and Bi) Nanoparticle-Decorated TiO2 Photocatalysts. Nanomaterials 2018, 8, 269. [Google Scholar] [CrossRef]
- Razzaq, A.; Grimes, C.A.; In, S.I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.; Razzaq, A.; Grimes, C.A.; In, S.I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded with graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of Visible Light Photocatalytic Graphene Embedded Rutile Titanium(IV) Oxide Composite Nanowires and Enhanced NOx Removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef]
- Park, S.M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.-I. Hybrid CuxO-TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef]
- Zubair, M.; Razzaq, A.; Grimes, C.A.; In, S.-I. Cu2ZnSnS4 (CZTS)-ZnO: A noble metal-free hybrid Z-scheme photocatalyst for enhanced solar-spectrum photocatalytic conversion of CO2 to CH4. J. CO2 Util. 2017, 20, 301–311. [Google Scholar] [CrossRef]
- In, S.-I.; Vaughn, D.D.; Schaak, R.E. Hybrid CuO-TiO2−xNx hollow nanocubes for photocatalytic conversion of CO2 into methane under solar irradiation. Angew. Chem. Int. Ed. 2012, 51, 3915–3918. [Google Scholar] [CrossRef] [PubMed]
- Parayil, S.K.; Razzaq, A.; In, S.-I. Formation of Titania-Silica Mixed Oxides in Solvent Mixtures and Their Influences for the Photocatalytic CO2 Conversion to Hydrocarbon. J. Nanosci. Nanotechnol. 2015, 15, 7285–7292. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Anpo, M.; Yamashita, H.; Ichihashi, Y.; Ehara, S. Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J. Electroanal. Chem. 1995, 396, 21–26. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Dimitrijevic, N.M.; Marin, T.W.; He, H.; Zapol, P. Heteroatom-transfer coupled photoreduction and carbon dioxide fixation on metal oxides. J. Phys. Chem. C 2012, 116, 9461–9471. [Google Scholar] [CrossRef]
- Tan, S.S.; Zou, L.; Hu, E. Kinetic modelling for photosynthesis of hydrogen and methane through catalytic reduction of carbon dioxide with water vapour. Catal. Today 2008, 131, 125–129. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. Computational screening of dopants for photocatalytic two-electron reduction of CO2 on anatase (101) surfaces. Energy Environ. Sci. 2012, 5, 6196. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, S.; Chen, R.; Zhu, X.; Liao, Q.; Huang, Y. Copper-decorated TiO2 nanorod thin films in optofluidic planar reactors for efficient photocatalytic reduction of CO2. Int. J. Hydrogen Energy 2017, 42, 9722–9732. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Luo, D.; Li, J.; Huang, Y.; Li, H.; Fang, Y.; Xu, Y.; Zhu, L. Adsorption of CO2 on heterostructure CdS(Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation. Chem. Eng. J. 2012, 180, 151–158. [Google Scholar] [CrossRef]
- Li, Q.; Zong, L.; Li, C.; Yang, J. Reprint of “Photocatalytic reduction of CO2 on MgO/TiO2 nanotube films”. Appl. Surf. Sci. 2014, 319, 16–20. [Google Scholar] [CrossRef]
- Qingli, W.; Zhaoguo, Z.; Xudong, C.; Zhengfeng, H.; Peimei, D.; Yi, C.; Xiwen, Z. Photoreduction of CO2 using black TiO2 films under solar light. J. CO2 Util. 2015, 12, 7–11. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Shen, X.; Peng, P.; Xiong, L.; Yu, Y. Octahedral Cu2O-modified TiO2 nanotube arrays for efficient photocatalytic reduction of CO2. Cuihua Xuebao Chin. J. Catal. 2015, 36, 2229–2236. [Google Scholar] [CrossRef]
- Liu, E.; Qi, L.; Bian, J.; Chen, Y.; Hu, X.; Fan, J.; Liu, H.; Zhu, C.; Wang, Q. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol. Mater. Res. Bull. 2015, 68, 203–209. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, S.; Chen, R.; Zhu, X.; Liao, Q.; Huang, Y. Visible light responsive CdS sensitized TiO2 nanorod array films for efficient photocatalytic reduction of gas phase CO2. Mol. Catal. 2018, 448, 185–194. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Song, M.; Li, D.; Zhang, X.; Liu, Y. TiO2−x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with H2O steam. Appl. Catal. B Environ. 2018, 243, 760–770. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Gold-nanoparticle-modified TiO2 nanowires for plasmon-enhanced photocatalytic CO2 reduction with H2 under visible light irradiation. Appl. Surf. Sci. 2015, 356, 1289–1299. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Zakaria, Z.Y. Photo-induced reduction of CO2 to CO with hydrogen over plasmonic Ag-NPs/TiO2 NWs core/shell hetero-junction under UV and visible light. J. CO2 Util. 2017, 18, 250–260. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Synergistic effect in plasmonic Au/Ag alloy NPs co-coated TiO2 NWs toward visible-light enhanced CO2 photoreduction to fuels. Appl. Catal. B Environ. 2017, 204, 548–560. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, C.Y.; Wu, R.J. Preparation of Pd/TiO2 nanowires for the photoreduction of CO2 into renewable hydrocarbon fuels. J. Taiwan Inst. Chem. Eng. 2019, 96, 409–418. [Google Scholar] [CrossRef]
- Song, G.; Xin, F.; Yin, X. Photocatalytic reduction of carbon dioxide over ZnFe2O4/TiO2 nanobelts heterostructure in cyclohexanol. J. Colloid Interface Sci. 2015, 442, 60–66. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Z.; Shen, X.; Dai, J.; Li, Y.; Li, Y. A comparative study on the photocatalytic behavior of graphene-TiO2 nanostructures: Effect of TiO2 dimensionality on interfacial charge transfer. Chem. Eng. J. 2018, 334, 907–921. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B Environ. 2019, 243, 522–536. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Godin, R.; Moss, B.; Kafizas, A.; Zafeiratos, S.; Durrant, J.R.; Petit, C. Titanium dioxide/carbon nitride nanosheet nanocomposites for gas phase CO2 photoreduction under UV-visible irradiation. Appl. Catal. B Environ. 2019, 242, 369–378. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B Environ. 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Qamar, S.; Lei, F.; Liang, L.; Gao, S.; Liu, K.; Sun, Y.; Ni, W.; Xie, Y. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, C.; Yang, P.; He, Y.; Feng, J.; Li, D. Synergetic promotional effect of oxygen vacancy-rich ultrathin TiO2 and photochemical induced highly dispersed Pt for photoreduction of CO2 with H2O. Appl. Catal. B Environ. 2019, 244, 919–930. [Google Scholar] [CrossRef]
- He, Z.; Tang, J.; Shen, J.; Chen, J.; Song, S. Enhancement of photocatalytic reduction of CO2 to CH4 over TiO2 nanosheets by modifying with sulfuric acid. Appl. Surf. Sci. 2016, 364, 416–427. [Google Scholar] [CrossRef]
- Alves Melo Júnior, M.; Morais, A.; Nogueira, A.F. Boosting the solar-light-driven methanol production through CO2 photoreduction by loading Cu2O on TiO2-pillared K2Ti4O9. Microporous Mesoporous Mater. 2016, 234, 1–11. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, K.Q.; Zhang, F.; Fu, X.; Xu, Y.J. Unveiling the interplay between light-driven CO2 photocatalytic reduction and carbonaceous residues decomposition: A case study of Bi2WO6-TiO2 binanosheets. Appl. Catal. B Environ. 2018, 237, 424–431. [Google Scholar] [CrossRef]
- Adekoya, D.O.; Tahir, M.; Amin, N.A.S. g-C3N4/(Cu/TiO2) nanocomposite for enhanced photoreduction of CO2 to CH3OH and HCOOH under UV/visible light. J. CO2 Util. 2017, 18, 261–274. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, D.; Wu, W.; Wang, D.; Gao, Z.; Xu, F.; Cao, K.; Jiang, K. Preparation of TiO2 microspheres with tunable pore and chamber size for fast gaseous diffusion in photoreduction of CO2 under simulated sunlight. J. Colloid Interface Sci. 2019, 539, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hashemizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic reduction of CO2 to hydrocarbons using bio-templated porous TiO2 architectures under UV and visible light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.; Ding, H.; Feng, J.; Zhang, J.Z.; Zhu, Y.; Hamid, S.; Bahnemann, D.W. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency. Appl. Catal. B Environ. 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Kim, H.R.; Razzaq, A.; Grimes, C.A.; In, S.I. Heterojunction p-n-p Cu2O/S-TiO2/CuO: Synthesis and application to photocatalytic conversion of CO2 to methane. J. CO2 Util. 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B Environ. 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Z.; Zhong, W.; Liu, J.; Li, J.; Duan, A.; Jiang, G. Synthesis of 3D ordered macroporous TiO2-supported Au nanoparticle photocatalysts and their photocatalytic performances for the reduction of CO2 to methane. Catal. Today 2015, 258, 319–326. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).