Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk
Abstract
:1. Introduction
2. Results
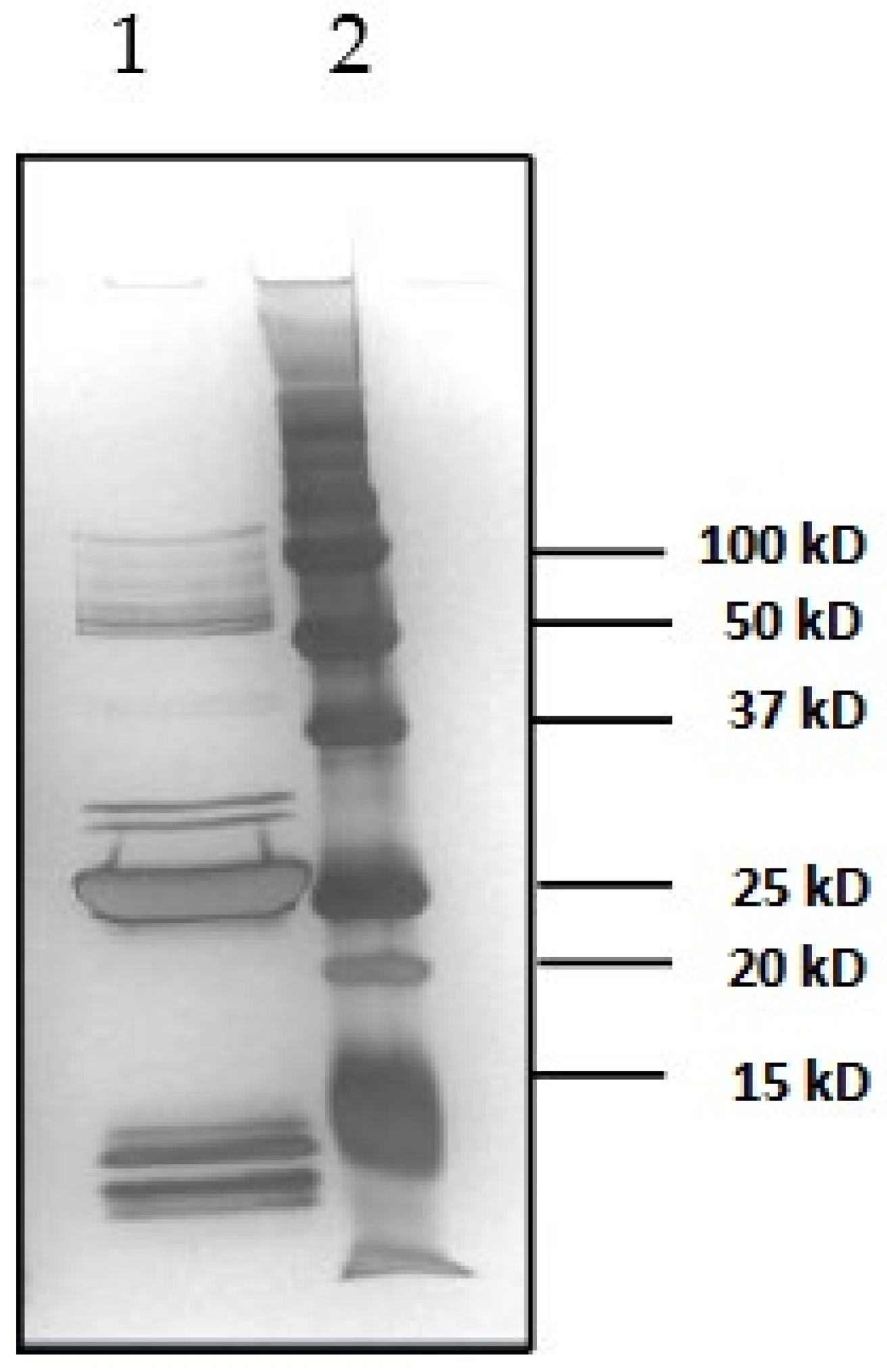
2.1. Characterization of Toxins
2.1.1. Determination of SEB Protein Concentration by in-House LC-ID-MS/MS AAA
2.1.2. Determination of SEB Protein Concentration by HPLC-FLD of OPA-Derivatized Amino Acids
2.1.3. Determination of SEA Protein Concentration by PSAQ Technology
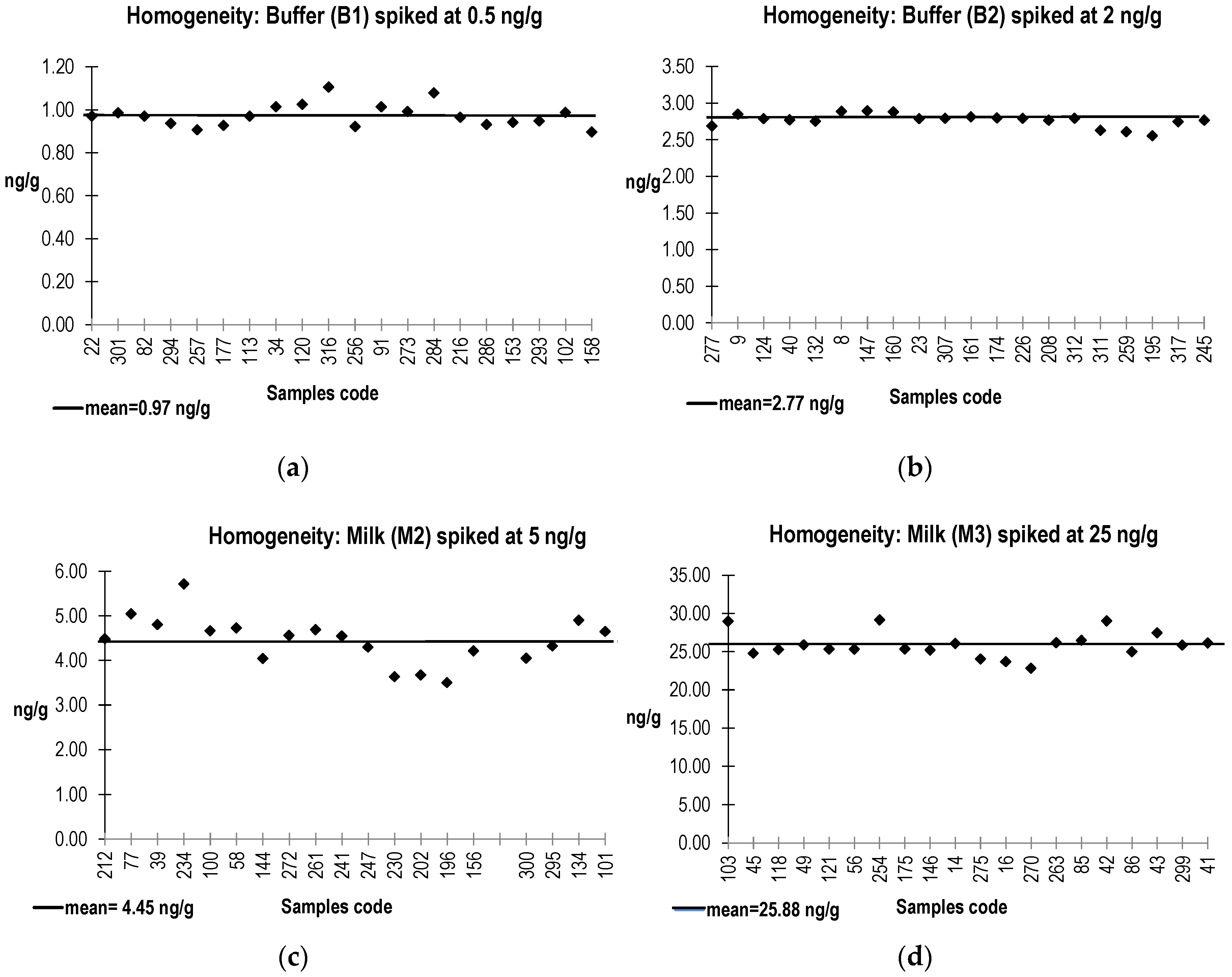
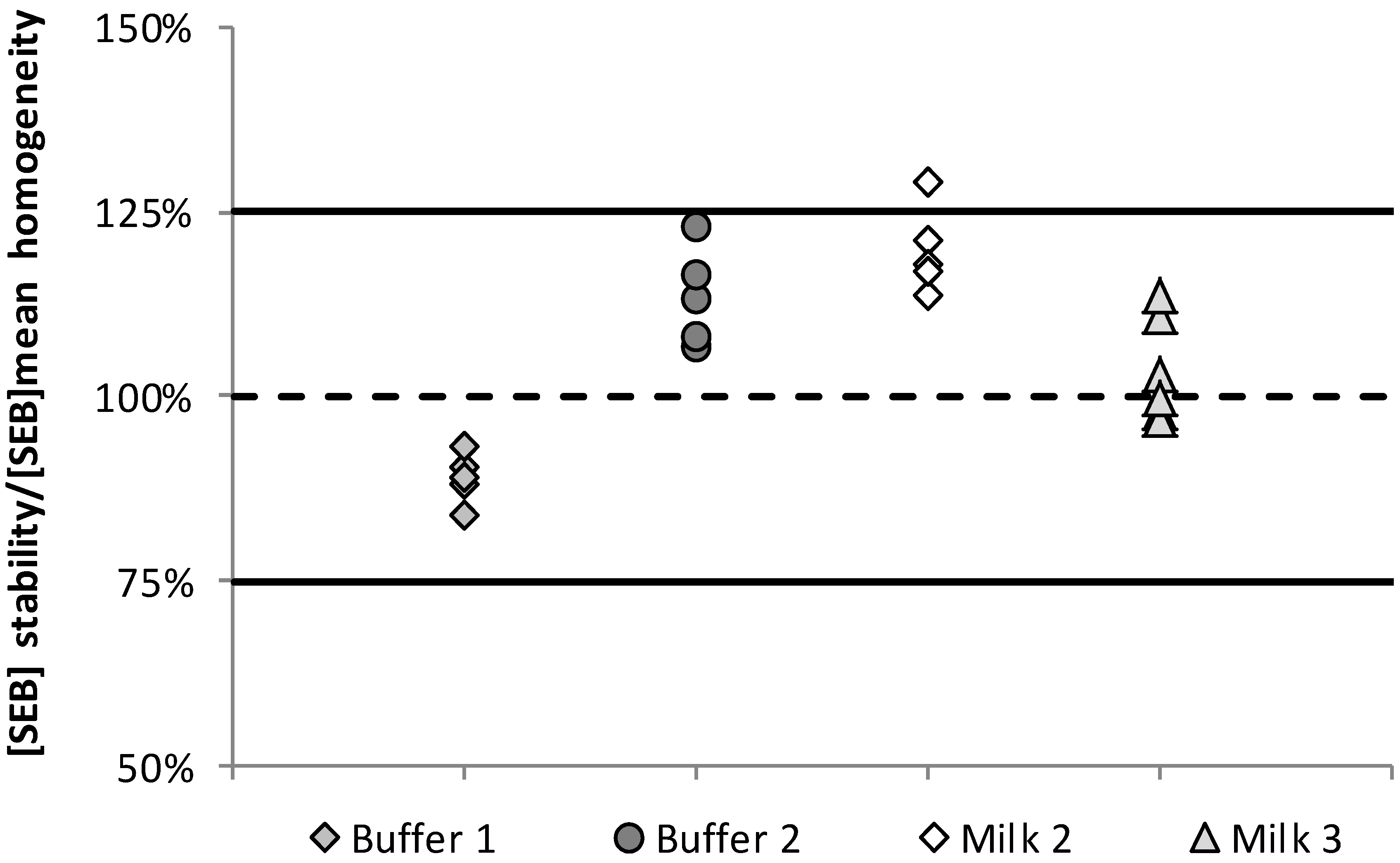
2.2. PT Materials Preparation, Homogeneity, and Stability Assessment
- (a)
- SEB in the six replicates dedicated to the stability test were well detected and quantified in buffer (B1 and B2) and milk (M1 and M2) samples, allowing the consideration that the samples were sufficiently stable when stored at 5 ± 3 °C over the PT period.
- (b)
- For material M4 spiked with SEA, SEB was not detected in the six replicates confirming the absence of cross reactions between the SEA and SEB using the in-house quantitative ELISA.
- (c)
- As in the homogeneity tests, six milk blank samples (M1) were analyzed by the ESM using the VIDAS SET2 detection assay. Data obtained showed that the five SE types (SEA, SEB, SEC, SED, and SEE) were not detected, confirming the absence of SEB external contamination in this blank material (M1).
2.3. PT Trial Organization
- (a)
- laboratories from the network were invited to participate the PT trial and received information on the dispatch of samples and the period dedicated for analyzing samples and reporting results (six weeks).17 participants confirmed their participation using the ELISA method, but 1 laboratory withdrew after receiving the samples. Therefore, 16 laboratories took parts in this PT trial using the ELISA methods.
- (b)
- samples were prepared (Section 5.3) and homogeneity test was performed on 20 replicates for each material.
- (c)
- (once the homogeneity was confirmed, samples were dispatched on the indicated period, but only to participants registered for the test.
- (d)
- after receiving the participant results, a stability test was performed for each material on the six replicates dedicated to this test (Section 5).
- (e)
- received data were assessed, a report was produced and participants received their individual results.
2.4. Results Obtained by ELISA-Based Methods
- (a)
- A green box: Staphylococcal enterotoxins were detected, the serotype SEB was identified in samples B1 and B2, M2 and M3. For M1 sample (blank), SEs were not detected especially SEB. For M4 sample (SEA spiked), SEB was not specifically detected.
- (b)
- A light green box: Staphylococcal enterotoxin type SEB was detected in B1, B2, M2, and M3 but other serotypes were identified in these samples. For M1 sample (blank) and M4 samples (SEA spiked), SEs were not detected without specification of the toxin type.
- (c)
- A red box: false positive or false negative results were obtained.
- (d)
- A white box: samples were not analyzed.
3. Discussion
- (a)
- enzyme immunoassay (EIA) comprising enzyme-linked immunosorbent assay (ELISA) and enzyme-linked fluorescent assay (ELFA).
- (b)
- reversed passive latex agglutination (RPLA).
4. Conclusions
5. Materials and Methods
5.1. Choice of Samples
- (a)
- Phosphate buffered saline (NaCl/Na2HPO4: 145 mM/10 mM, Sigma-Aldrich (St. Quentin Fallavier, France)) at pH 7.3 ± 0.2 was considered as an easy to use matrix.
- (b)
- As the EU regulation 2073/2005 modified by the European regulation 1441/2007 [21] enforces testing of SEs content in milk and milk based products, it was decided to use liquid UHT fat-free milk as a complex matrix (purchased from a retail store).
5.2. Characterization of Toxins
5.2.1. Determination of Protein Concentration of SEB by Amino Acid Analysis (AAA)
- (a)
- SGS M-Scan (SGS M-Scan; Freiburg, Germany) [23]. Briefly, Norvaline and Sarcosine were added to SEB solution as internal standards for primary and secondary amino acids and hydrolyzed in 6 M HCl at 110 °C for 24 h. Then, the obtained solution was analyzed by HPLC with fluorescence detection following pre-column derivatization with OPA (ortho-phthalaldehyde) with fluorescence detection at 340/450 nm for excitation and emission, respectively. For calculation of protein concentrations, only robust amino acids were taken into account as follows;
- (b)
- JRC-IRMM, Geel, Belgium (from EQuATox network). AAA was carried out as described in detail in the laboratory working instruction of JRC-IRMM and is based on the publication by Muñoz et al. [11]. Briefly, the protein sample is hydrolyzed using 6 M HCl and 0.1% of phenol using a 1 h microwave digestion protocol. Liberated amino acids were separated on a reverse-phase HPLC column and quantified by tandem mass spectrometry using pure amino acids for calibration and isotopically labelled amino acids as internal standards. Ala, Pro, Val, Leu, Iso, and Phe are the amino acids which are most stable during hydrolysis and resistant to oxidation under the conditions employed; these were thus quantified and the results converted into protein concentration using the published SEB protein sequence [22]. Some deviations from the validated method were made, for instance: volumetric instead of gravimetric sample preparation due to safety reasons; employment of lower volumes of toxin, buffer, and internal standard solutions to allow six independent analyses; and the omission of a so-called sample blank (sample with all solvents, reagents and internal standards, but without hydrolysis, again for safety reasons).
5.2.2. Determination of Protein Concentration of SEA by PSAQ Technology
5.3. Preparation of Samples
- (a)
- blank milk sample (M1),
- (b)
- two milk samples spiked with SEB at 5 ng/g (M2) and 25 ng/g (M3),
- (c)
- one milk sample spiked with SEA at 10 ng/g (M4),
- (d)
- two buffer samples spiked with SEB at 0.5 ng/g (B1) and 2 ng/g (B2) (Figure 4).
- (a)
- homogeneity (20 samples for each material) and stability studies (6 samples for each material),
- (b)
- dispatch samples dedicated to participants,
- (c)
- additional samples in case of need (10 samples for each material).
5.4. Homogeneity Study and Determination of Assigned Values
- (a)
- the quantitative ELISA (M2, M3, and M4), which allowed to estimate the concentration of SEB in M2 and M3 samples, and to check cross reaction in the case of M4 sample (spiked by SEA).
- (b)
5.5. Stability Study of Batches
5.6. Organization of Proficiency Testing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The European Food Safety Authority; The European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J. 2012, 2559, 1–442. [Google Scholar]
- The European Food Safety Authority; The European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J. 2013, 3129, 1–250. [Google Scholar]
- The European Food Safety Authority; The European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. 2014, 3547, 1–312. [Google Scholar]
- The European Food Safety Authority; The European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015, 3991, 1–165. [Google Scholar]
- The European Food Safety Authority; The European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 4329, 1–191. [Google Scholar]
- Moran, G.J. Threats in bioterrorism II: CDC category B and C agents. Emerg. Med. Clin. N. Am. 2002, 20, 311–330. [Google Scholar] [CrossRef]
- Croddy, E.C.; Perez-Armendariz, C.; Hart, J. Chemical and Biological Warfare: A Comprehensive Survey for the Concerned Citizen; Springer-Verlag: New York, NY, USA, 2002; pp. 30–31. [Google Scholar]
- Ulrich, R.G. Staphylococcal enterotoxin B and related pyrogenic toxins. In Textbook of Military Medicine; Warfare, Weaponry and the Casualty, U.S. Government Printing Office: Washington, DC, USA, 1997; Part I; Volume 3, pp. 621–631. [Google Scholar]
- Regulation (EC) N°882/2004 of the European Parliament and Council of 29 April 2004 on Official Controls Performed to Ensure the Verification of Compliance with Feed and Food Law, Animal Health and Animal Welfare Rules. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02004R0882-20120101 (accessed on 5 September 2016).
- Establishment of Quality Assurances for the Detection of Biological Toxins of Potential Bioterrorism Risk Network. Available online: http://www.equatox.eu/ (accessed on 8 September 2016).
- Munoz, A.; Kral, R.; Schimmel, H. Quantification of protein calibrants by amino acid analysis using isotope dilution mass spectrometry. Anal. Biochem. 2011, 408, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Brun, V.; Dupuis, A.; Adrait, A.; Marcellin, M.; Thomas, D.; Court, M.; Vandenesch, F.; Garin, J. Isotope-labeled protein standards: Toward absolute quantitative proteomics. Mol. Cell Proteom. 2007, 6, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Kirchner, S.; Stoermann, B.; Schreiber, T.; Kaulfuss, S.; Schade, R.; Zbinden, R.; Avondet, M.A.; Dorner, M.B.; Dorner, B.G. Simultaneous quantification of five bacterial and plant toxins from complex matrices using a multiplexed fluorescent magnetic suspension assay. Analyst 2009, 134, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Dorner, B.G.; Zeleny, R.; Harju, K.; Hennekinne, J.A.; Vanninen, P.; Schimmel, H.; Rummel, A. Biological toxins of potential bioterrorism risk: Current status of detection and identification technology. Trends Anal. Chem. 2016, in press. [Google Scholar] [CrossRef]
- Method of Detection and Quantification of Staphylococcal Enterotoxins Types Sea to See in All Types of Food Matrices by the Elisa Method after Extraction and Dialysis Concentration (Confirmatory Method of the Eurl for CPS, v1, July 2012). Available online: https://eurl-staphylococci.anses.fr/en/minisite/staphylococci/european-union-reference-laboratory-staphylococci (accessed on 5 September 2016).
- Mamone, G.; Picariello, S.; Caira, F.; Addeo, P.; Ferranti, P. Analysis of food proteins and peptides by mass spectrometry based techniques. J. Chromatogr. A 2009, 1216, 7130–7142. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, M.; Tsilia, V.; Rajkovic, A.; de Cremer, K.; van Loco, J. Application of LC-MS/MS MRM to Determine Staphylococcal Enterotoxins (SEB and SEA) in Milk. Toxins 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Nia, Y.; Mutel, I.; Assere, A.; Lombard, B.; Auvray, F.; Hennekinne, J.A. Review over a 3-Year Period of European Union Proficiency Tests for Detection of Staphylococcal Enterotoxins in Food Matrices. Toxins 2016, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Zeleny, R.; Emteborg, H.; Charoud-Got, J.; Schimmel, H.; Nia, Y.; Mutel, I.; Ostyn, A.; Herbin, S.; Hennekinne, J.A. Development of a reference material for Staphylococcus aureus enterotoxin A in cheese: Feasibility study, processing, homogeneity and stability assessment. Food Chem. 2015, 168, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; Ostyn, A.; Guillier, F.; Herbin, S.; Prufer, A.L.; Dragacci, S. How should staphylococcal food poisoning outbreaks be characterized? Toxins 2010, 2, 2106–2116. [Google Scholar] [CrossRef] [PubMed]
- The Commision of the European Communities. Commission Regulation (EC) No. 1441/2007 of 5 December 2007 amending Regulation (EC) No. 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2007, L322, 12–29. [Google Scholar]
- Jones, C.L.; Khan, S.A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J. Bacteriol. 1986, 166, 29–33. [Google Scholar]
- SGS laboratory (Protein and Peptide Analysis). Available online: http://www.sgs.com/en/life-sciences/biopharmaceutical-services/laboratory-services/protein-and-peptide-analysis (accessed on 8 September 2016).
- Dupuis, A.; Hennekinne, J.A.; Garin, J.; Brun, V. Protein Standard Absolute Quantification (PSAQ) for improved investigation of staphylococcal food poisoning outbreaks. Proteomics 2008, 8, 4633–4636. [Google Scholar] [CrossRef]
- ISO/IEC 17043:2010. Conformity Assessment-General Requirements for Proficiency Testing. Available online: http://www.iso.org/iso/catalogue_detail?csnumber=29366 (accessed on 5 September 2016).
- EN ISO 13528:2005. Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparisons. Available online: http://www.cpts.org.cn/UploadFiles/PTjs/2011/11/20111114142812.pdf (accessed on 5 September 2016).
- ISO/IEC 17025:2005. General Requirements for the Competence of Testing and Calibration Laboratories; International Standard Organization (ISO). Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=39883 (accessed on 5 September 2016).
- Hennekinne, J.A.; Guillier, F.; Perelle, S.; de Buyser, M.L.; Dragacci, S.; Lombard, B.; Krys, S. Intra-laboratory validation of the Vidas SET2 detection kit in milk products according to the EN ISO 16 140 standard. J. Appl. Microbiol. 2007, 102, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; Ostyn, A.; Guillier, F.; Gohier, M.; Messio, S.; Dragacci, S.; Krys, S.; Lombard, B. Interlaboratory validation of the Vidas SET2 detection kit for an use in official controls of staphylococcal enterotoxins detection in milk products especially low-fat cheeses. Int. J. AOAC 2007, 90, 756–764. [Google Scholar]

| Lab Code | Method Used | M1 | M2 | M3 | M4 | B1 | B2 | |
|---|---|---|---|---|---|---|---|---|
| 1 | ELISA electrochemical immunosensor—Portable Toxin Detector pTD (Bruker) | |||||||
| 3 | Ridascreen SET ABCDE (Rbiopharm) | |||||||
| In house ELISA | ||||||||
| 4 | Vidas SET2 (Biomerieux) | |||||||
| 5 | Oxoid SET RPLA (Termo Scientific) | |||||||
| 6 | Vidas SET2 (Biomerieux) | |||||||
| Oxoid SET RPLA (Termo Scientific) | ||||||||
| TetraCore ELISA kit (TetraCore) | ||||||||
| 7 | miPROTECT SEB (Miprolab) | |||||||
| 8 | In house ELISA | |||||||
| Western blot | ||||||||
| 9 | In house ELISA [13,14] | extraction without dialysis concentration | ||||||
| extraction with dialysis concentration | ||||||||
| 10 | miPROTECT SEB (Miprolab) | |||||||
| 11 | Vidas SET2 (Biomerieux) | |||||||
| Oxoid SET RPLA (Termo Scientific) | ||||||||
| 12 | Vidas SET2 (Biomerieux) | |||||||
| Tecra SET VIA (3M) | ||||||||
| Ridascreen SET Total (Rbiopharm) | ||||||||
| In house ELISA [15] | ||||||||
| 13 | Vidas SET2 (Biomerieux) | |||||||
| Oxoid SET RPLA (Termo Scientific) | ||||||||
| 14 | Ridascreen SET ABCDE (Rbiopharm) | |||||||
| TetraCore ELISA kit (TetraCore) | ||||||||
| In house ELISA | ||||||||
| 16 | Lateral Flow Assay | |||||||
| 17 | In house EIA | |||||||
| In house Immunochromatographic Test SEB | ||||||||
| Lab Code | Method Used | M2 | M3 | B1 | B2 | |
|---|---|---|---|---|---|---|
| 1 | ELISA electrochemical immunosensor—Portable Toxin Detector pTD (Bruker) | |||||
| 3 | In house ELISA | |||||
| 7 | miPROTECT SEB (Miprolab) | |||||
| 8 | In house ELISA | |||||
| 9 | In house ELISA [13,14] | extraction without dialysis concentration | ||||
| extraction with dialysis concentration | ||||||
| 12 | In-house-ELISA [15] | |||||
| 14 | In-house-ELISA | |||||
| 16 | Lateral Flow Assay | |||||
| 17 | In house EIA | |||||
| 17 | In house Immunochromatographic Test SEB | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nia, Y.; Rodriguez, M.; Zeleny, R.; Herbin, S.; Auvray, F.; Fiebig, U.; Avondet, M.-A.; Munoz, A.; Hennekinne, J.-A. Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk. Toxins 2016, 8, 268. https://doi.org/10.3390/toxins8090268
Nia Y, Rodriguez M, Zeleny R, Herbin S, Auvray F, Fiebig U, Avondet M-A, Munoz A, Hennekinne J-A. Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk. Toxins. 2016; 8(9):268. https://doi.org/10.3390/toxins8090268
Chicago/Turabian StyleNia, Yacine, Mélanie Rodriguez, Reinhard Zeleny, Sabine Herbin, Frédéric Auvray, Uwe Fiebig, Marc-André Avondet, Amalia Munoz, and Jacques-Antoine Hennekinne. 2016. "Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk" Toxins 8, no. 9: 268. https://doi.org/10.3390/toxins8090268
APA StyleNia, Y., Rodriguez, M., Zeleny, R., Herbin, S., Auvray, F., Fiebig, U., Avondet, M.-A., Munoz, A., & Hennekinne, J.-A. (2016). Organization and ELISA-Based Results of the First Proficiency Testing to Evaluate the Ability of European Union Laboratories to Detect Staphylococcal Enterotoxin Type B (SEB) in Buffer and Milk. Toxins, 8(9), 268. https://doi.org/10.3390/toxins8090268





