Characterization of Enzymatic Activity of MlrB and MlrC Proteins Involved in Bacterial Degradation of Cyanotoxins Microcystins
Abstract
:1. Introduction
2. Results
2.1. Verification of Nucleotide Sequence Coding for MlrB
2.2. Recombinant MlrB, MlrC, and Their Mutants
2.3. MlrB and MlrC Purification
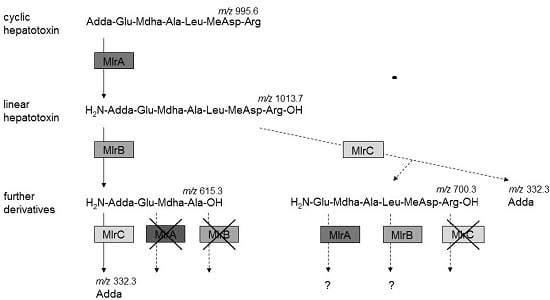
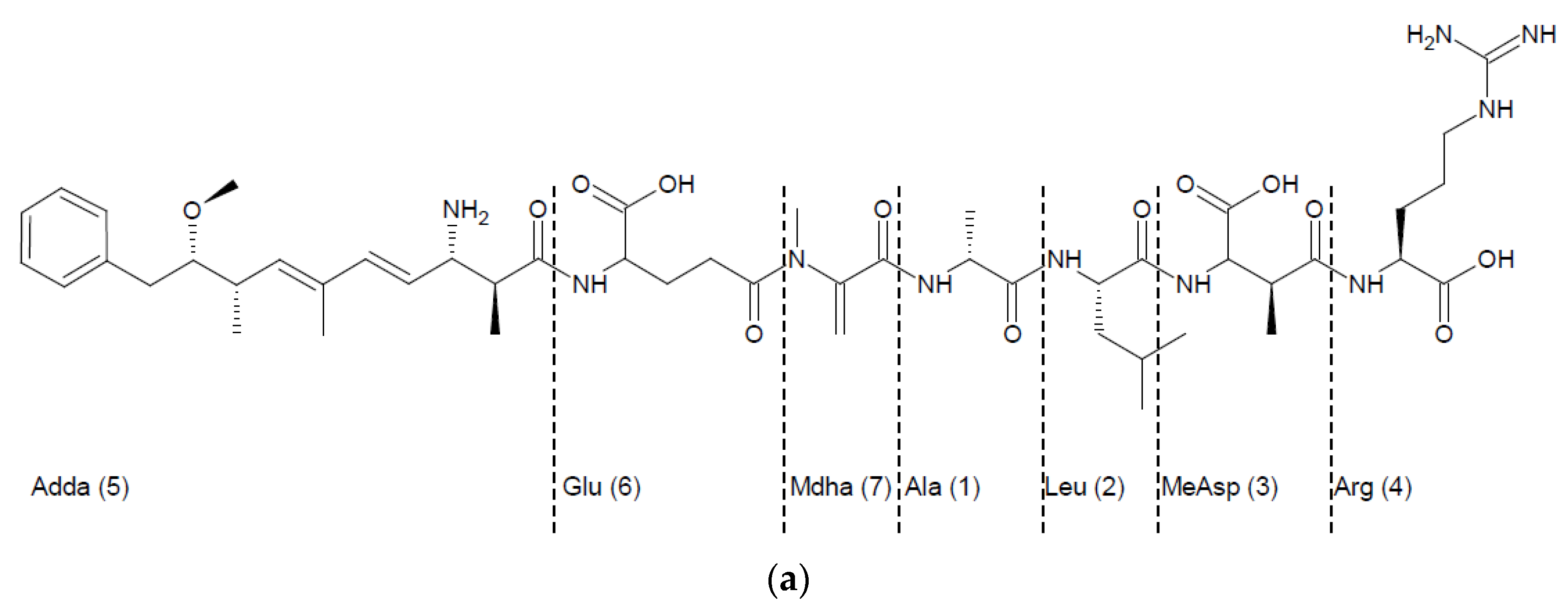
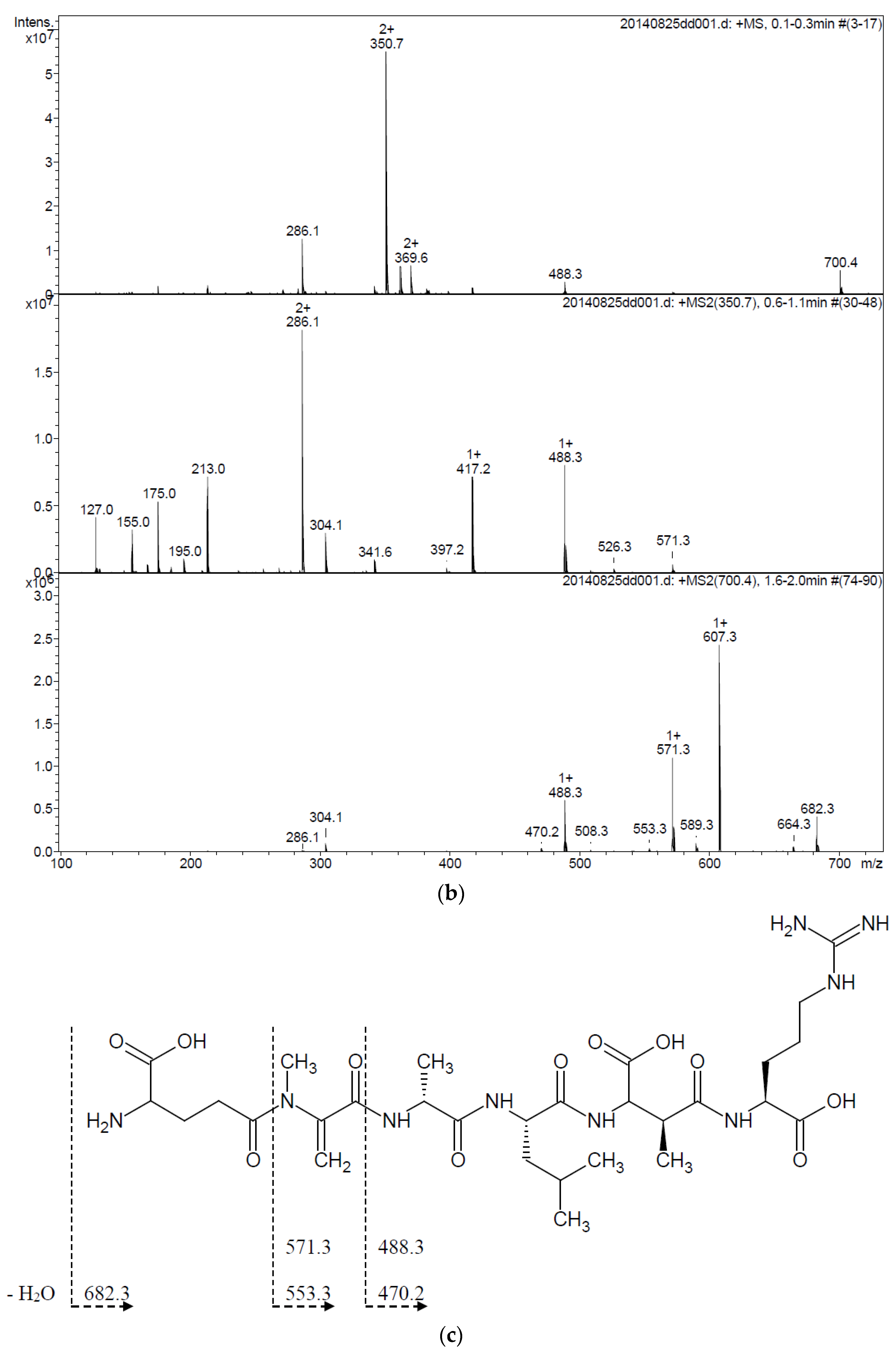
2.4. Activity of Recombinant MlrA, MlrB, and MlrC toward MC Derivatives
3. Discussion
3.1. Recombinant MlrB and MlrC—Verification of Sequence, Construction, and Analysis of Muteins, Purification
3.2. MCs Degradation Pathway
4. Materials and Methods
4.1. Chemicals and Bacterial Strains
4.2. Verification of MlrB Coding Sequence
4.3. Construction of Recombinant Plasmids, Including Mlrb and Mlrc and Their Mutants
4.4. Expression of Recombinant MlrB and MlrC
4.5. Purification of Recombinant MlrB and MlrC
4.6. Activity Assays
4.7. HPLC and Mass Spectrometry
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| acMCs | (linearized) acyclic microcystins |
| Adda | 3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-deca-4,6-dienoic acid |
| BLAST | Basic Local Alignment Search Tool |
| Co-NTA | Ni-NTA column with nickel ions replaced with cobalt ions |
| hexaMCs | hexapeptides produced from acyclic MCs |
| HPLC | high performance liquid chromatography |
| IPTG | isopropyl β-d-1-thiogalactopyranoside |
| m/z | mass to charge ratio |
| MC | microcystin |
| Mdha | methyldehydroalanine |
| NOD | nodularin |
| ORFs | open reading frames |
| PCR | polymerase chain reaction |
| PMSF | phenylmethanesulfonyl fluoride |
References
- Dziga, D.; Wasylewski, M.; Wladyka, B.; Nybom, S.; Meriluoto, J. Microbial degradation of microcystins. Chem. Res. Toxicol. 2013, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.G.; Jones, G.J.; Blakeley, R.L.; Jones, A.; Negri, A.P.; Riddles, P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Appl. Environ. Microbiol. 1996, 62, 4086–4094. [Google Scholar] [PubMed]
- Bourne, D.G.; Riddles, P.; Jones, G.J.; Smith, W.; Blakeley, R.L. Characterization of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environ. Toxicol. 2001, 16, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Manage, P.M.; Edwards, C.; Singh, B.K.; Lawton, L.A. Isolation and identification of novel microcystin-degrading bacteria. Appl. Environ. Microbiol. 2009, 75, 6924–6928. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Yang, J.D.; Zhou, W.; Yin, Y.F.; Chen, J.; Shi, Z.Q. Isolation of a Methylobacillus sp. that degrades microcystin toxins associated with cyanobacteria. New Biotechnol. 2009, 26, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Nybom, S.M.K.; Dziga, D.; Heikkilä, J.E.; Kull, T.P.J.; Salminen, S.J.; Meriluoto, J.A.O. Characterization of microcystin-LR removal process in the presence of probiotic bacteria. Toxicon 2012, 59, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ame, M.V.; Echenique, J.R.; Pflugmacher, S.; Wunderlin, D.A. Degradation of microcystin-RR by Sphingomonas sp CBA4 isolated from San Roque reservoir (Cordoba—Argentina). Biodegradation 2006, 17, 447–455. [Google Scholar]
- Edwards, C.; Graham, D.; Fowler, N.; Lawton, L.A. Biodegradation of microcystins and nodularin in freshwaters. Chemosphere 2008, 73, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Wladyka, B.; Zielińska, G.; Meriluoto, J.; Wasylewski, M. Heterologous expression and characterisation of microcystinase. Toxicon 2012, 59, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Shimizu, K.; Kawauchi, Y.; Maseda, H.; Utsumi, M.; Zhang, Z.; Neilan, B.A.; Sugiura, N. Characteristics of a microcystin-degrading bacterium under alkaline environmental conditions. J. Toxicol. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, P.; Chen, J.; Yan, H. Biodegradation of microcystin-RR by a new isolated Sphingopyxis sp. USTB-05. Chin. J. Chem. Eng. 2010, 18, 1–5. [Google Scholar] [CrossRef]
- Szweda, P.; Kotłowski, R.; Kur, J. New effective sources of the Staphylococcus simulans lysostaphin. J. Biotechnol. 2005, 117, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Kato, H.; Mizuno, M.; Tsuji, K.; Harada, K. Bacterial degradation of microcystins and nodularin. Chem. Res. Toxicol. 2005, 18, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Maseda, H.; Okano, K.; Kurashima, T.; Kawauchi, Y.; Xue, Q.; Utsumi, M.; Zhang, Z.; Sugiura, N. Enzymatic pathway for biodegrading microcystin LR in Sphingopyxis sp. C-1. J. Biosci. Bioeng. 2012, 114, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Bohuslavek, J.; Payne, J.W.; Liu, Y.; Bolton, H., Jr.; Xun, L. Cloning, sequencing, and characterization of a gene cluster involved in EDTA degradation from the bacterium BNC1. Appl. Environ. Microbiol. 2001, 67, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Imanishi, S.; Kato, H.; Mizuno, M.; Ito, E.; Tsuji, K. Isolation of Adda from microcystin-LR by microbial degradation. Toxicon 2004, 44, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Imanishi, S.Y.; Tsuji, K.; Harada, K. Microbial degradation of cyanobacterial cyclic peptides. Water Res. 2007, 8, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Wasylewski, M.; Szetela, A.; Bocheńska, O.; Wladyka, B. Verification of the role of MlrC in microcystin biodegradation by studies using a heterologously expressed enzyme. Chem. Res. Toxicol. 2012, 25, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, E.H.; Kato, H.; Kawasaki, Y.; Nozawa, Y.; Tsuji, K.; Hirooka, E.Y.; Harada, K. Further investigation of microbial degradation of microcystin using the advanced Marfey method. Chem. Res. Toxicol. 2009, 22, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.I. Chemistry and detection of microcystins. In Toxic Microcystis; Watanabe, M.F., Harada, K.I., Carmichael, W.W., Fujiki, H., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 103–148. [Google Scholar]
- Shimizu, K.; Maseda, H.; Okano, K.; Itayama, T.; Kawauchi, Y.; Chen, R.; Utsumi, M.; Zhang, Z.; Sugiura, N. How microcystin-degrading bacterium express degradation activity. Lakes Reserv. Res. Manag. 2011, 16, 169–178. [Google Scholar]
- Gajdek, P.; Lechowski, Z.; Dziga, D.; Bialczyk, J. Detoxification of microcystin-LR using Fenton reagent. Fresenius Environ. Bull. 2003, 12, 1258–1262. [Google Scholar]
- Meriluoto, J.; Spoof, L. Purification of microcystins by high-performance liquid chromatography. In Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Codd, G.A., Eds.; Åbo Akademi University Press: Turku, Finland, 2005; pp. 93–104. [Google Scholar]
- Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]


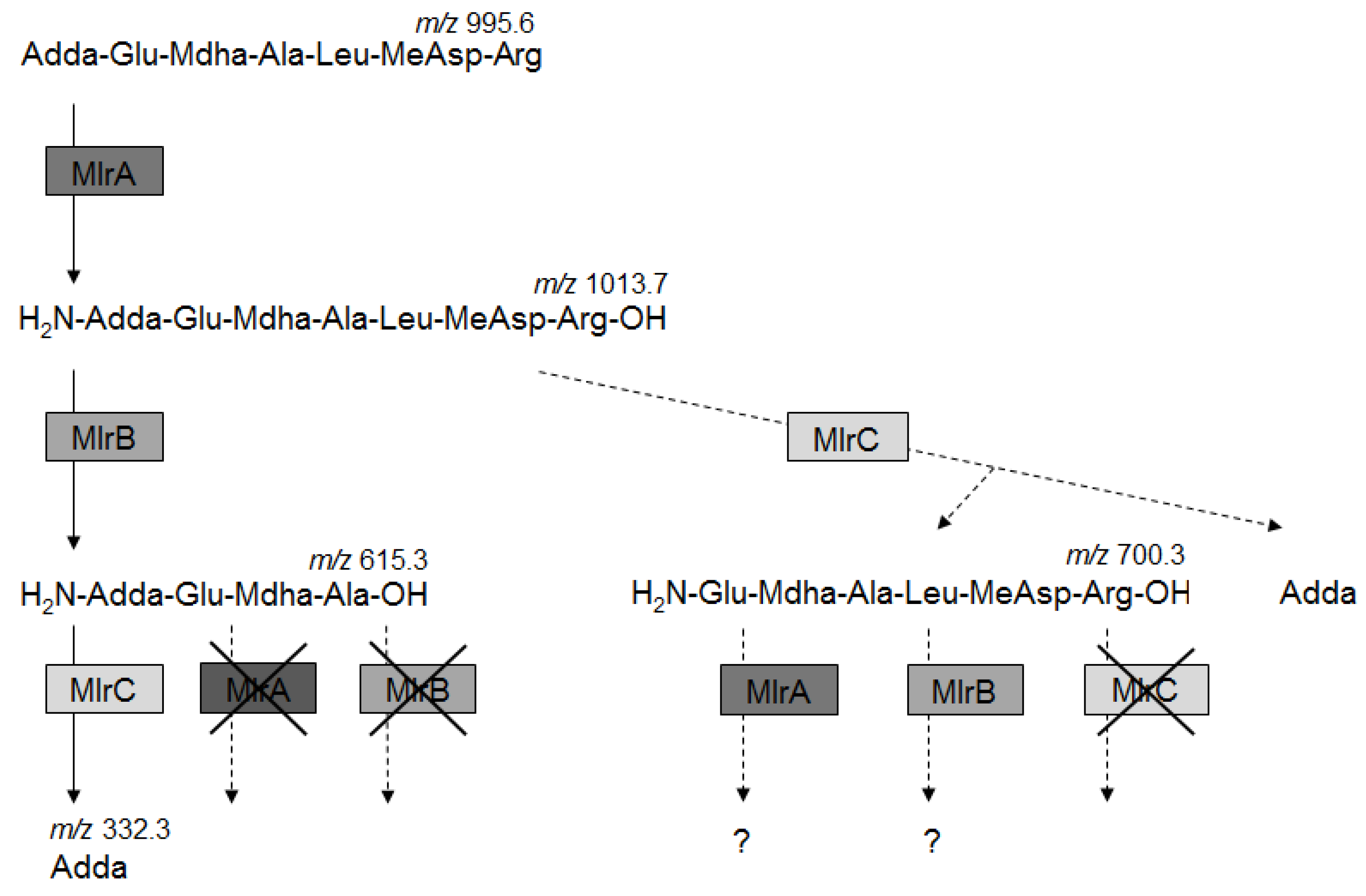
| MC Derivatives | MlrA | MlrAH260A | MlrB | MlrBS77A | MlrC | MlrCH169A | * Empty Plasmids |
|---|---|---|---|---|---|---|---|
| acMC-LR | − | − | + | − | + | − | − |
| acdmMC-LR | − | − | n.a. | − | + | − | − |
| acMC-LF | − | − | n.a. | − | + | − | − |
| acMC-LW | − | − | n.a. | − | + | − | − |
| acMC-LY | − | − | n.a. | − | + | − | − |
| acMC-RR | − | − | + | − | + | − | − |
| acMC-YR | − | − | n.a. | − | + | − | − |
| hexaMC-LR | + | − | + | − | − | − | − |
| hexaMC-RR | + | − | + | − | − | − | − |
| tetrapeptide | − | − | − | − | + | − | − |
| Linearized Variants of Different MC | MC-LR | dmMC-LR | MC-LW | MC-LF | MC-RR | MC-YR | MC-LY |
|---|---|---|---|---|---|---|---|
| m/z of heptapeptides | 1013.7 | 999.7 | 1043.6 | 1004.6 | 1056.5 528.9 * | 1063.7 | 1020.6 |
| retention time (min) | 5.4 | 5.3 | 6.7 | 6.6 | 4.6 | 5.1 | 6.1 |
| m/z of hexapeptides | 700.4 350.7 * | 686.4 343.7 * | 730.4 | 691.4 | 372.3 * | 750.4 375.7 * | 707.4 |
| retention time (min) | 2.7 | 2.7 | 4.7 | 4.7 | 1.7 | 2.6 | 3.8 |
| Primer Name | Sequence (5′ to 3′) | Amplified Fragment Length (nt) |
|---|---|---|
| InvBF | CAAAGCCGCCCTGAAAAAGAAC | - |
| InvBR | TATGCCGGTGGATTGTTCGTC | |
| mlrBF | AGAAGGAGATATAACTATGACTGCAACAAAGCTTTTCCTGGCG | 1660 |
| mlrBR | GTGGTGGTGATGGTGATGGCCTCGAAGCCGCCTGAACACTATCCCGTTCAG | |
| mlrBS77AF | CTTCGAGTTGGCGGCAACATCGAAGC | ~6124 * |
| mlrBS77AR | GCTTCGATGTTGCCGCCAACTCGAAG | |
| mlrBK80AF | CGTCAACATCGGCGCAGTTTACAGC | ~6124 * |
| mlrBK80AR | GCTGTAAACTGCGCCGATGTTGACG | |
| mlrCF | GTTCCATATGCTTGATCGTCGAACATTG | 1577 |
| mlrCR | GAAAGCGGCCGCGACAGGCTCGAATGGCCAC | |
| mlrCD167AF | GGGGCCGAACTTGCTCTTCACGCTCAC | 6947 * |
| mlrCD167AR | GTGAGCGTGAAGAGCAAGTTCGGCCCC | |
| mlrCH169AF | GAACTTGATCTTGCCGCTCACTTGTCG | 6947 * |
| mlrCH169AR | CGACAAGTGAGCGGCAAGATCAAGTTC | |
| mlrCH191AF | CAAGTACTATCCGGCTATCGACTACGTC | 6947 * |
| mlrCH191AR | GACGTAGTCGATAGCCGGATAGTACTTG |
| Protein | Substituted | Introduced | ||
|---|---|---|---|---|
| Residue | Codon | Residue | Codon | |
| MlrB | S77 | TCA | A77 | GCA |
| K80 | AAG | A80 | GCG | |
| MlrC | H167 | GAT | A167 | GCT |
| D169 | CAC | A169 | GCC | |
| H191 | CAT | H191 | GCT | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziga, D.; Zielinska, G.; Wladyka, B.; Bochenska, O.; Maksylewicz, A.; Strzalka, W.; Meriluoto, J. Characterization of Enzymatic Activity of MlrB and MlrC Proteins Involved in Bacterial Degradation of Cyanotoxins Microcystins. Toxins 2016, 8, 76. https://doi.org/10.3390/toxins8030076
Dziga D, Zielinska G, Wladyka B, Bochenska O, Maksylewicz A, Strzalka W, Meriluoto J. Characterization of Enzymatic Activity of MlrB and MlrC Proteins Involved in Bacterial Degradation of Cyanotoxins Microcystins. Toxins. 2016; 8(3):76. https://doi.org/10.3390/toxins8030076
Chicago/Turabian StyleDziga, Dariusz, Gabriela Zielinska, Benedykt Wladyka, Oliwia Bochenska, Anna Maksylewicz, Wojciech Strzalka, and Jussi Meriluoto. 2016. "Characterization of Enzymatic Activity of MlrB and MlrC Proteins Involved in Bacterial Degradation of Cyanotoxins Microcystins" Toxins 8, no. 3: 76. https://doi.org/10.3390/toxins8030076
APA StyleDziga, D., Zielinska, G., Wladyka, B., Bochenska, O., Maksylewicz, A., Strzalka, W., & Meriluoto, J. (2016). Characterization of Enzymatic Activity of MlrB and MlrC Proteins Involved in Bacterial Degradation of Cyanotoxins Microcystins. Toxins, 8(3), 76. https://doi.org/10.3390/toxins8030076