Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates
Abstract
:1. Introduction
2. Results
2.1. Immunization and Characterization of Elicited Antibodies
2.2. Isolation of Anti-Ricin Antibodies
2.3. Epitope Binning
2.4. In Vitro Neutralization of Ricin Cytotoxicity
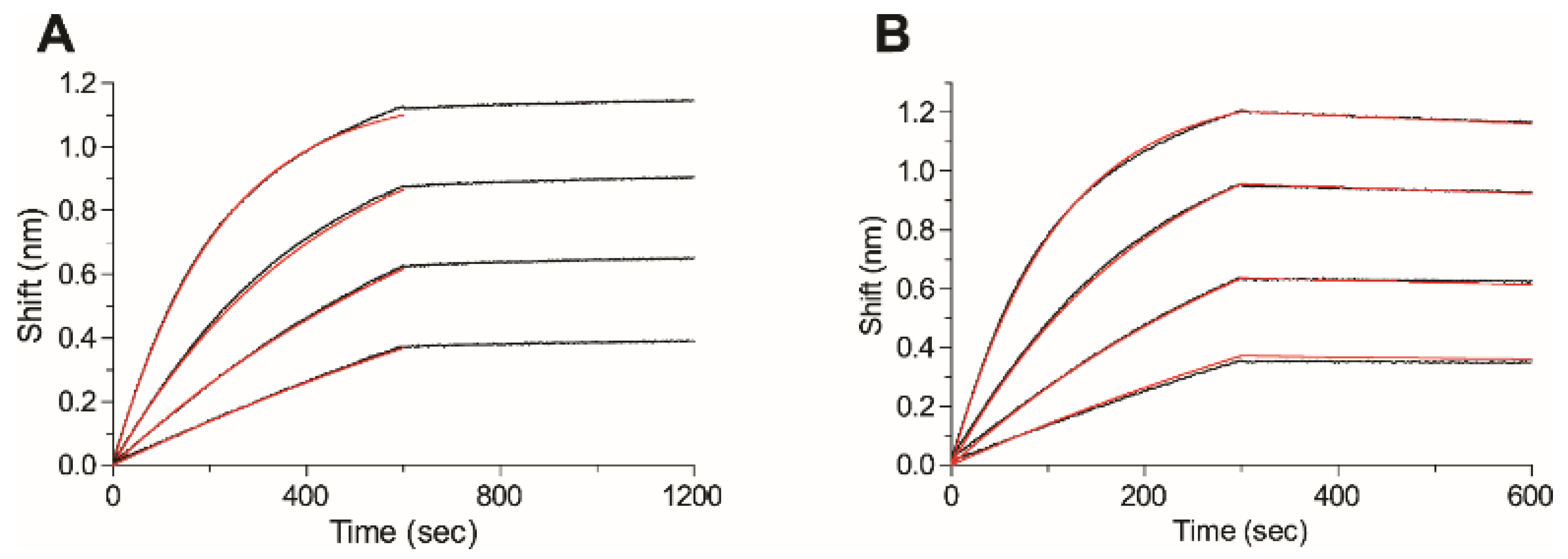
2.5. Affinity of the Anti-Ricin Antibodies
2.6. Post-Exposure Treatment of Ricin Intoxication
3. Discussion
4. Experimental Section
4.1. Animal Immunization
4.2. Primers Design
4.3. scFv Library Construction
4.4. Library Screening
4.5. ELISA
4.6. Fingerprint Analysis
4.7. Nucleic Acid Analysis
4.8. Producing Full-Length Antibodies
4.9. Affinity Measurements and Epitope Binning
4.10. In Vitro Ricin Neutralization Assay
4.11. In Vivo Protection Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsnes, S.; Kozlov, J.V. Ricin. Toxicon 2001, 39, 1723–1728. [Google Scholar] [CrossRef]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [PubMed]
- Spooner, R.A.; Smith, D.C.; Easton, A.J.; Roberts, L.M.; Lord, J.M. Retrograde transport pathways utilised by viruses and protein toxins. Virol. J. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. The RNA N-glycosidase activity of ricin A-chain. Nucleic Acids Symp. Ser. 1988, 19, 139–142. [Google Scholar] [PubMed]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Carra, J.H.; Wannemacher, R.W.; Tammariello, R.F.; Lindsey, C.Y.; Dinterman, R.E.; Schokman, R.D.; Smith, L.A. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine 2007, 25, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.J.; Brey, R.N.; Mantis, N.J.; Mapes, K.; Pop, I.V.; Pop, L.M.; Ruback, S.; Killeen, S.Z.; Doyle-Meyers, L.; Vinet-Oliphant, H.; et al. Thermostable ricin vaccine protects rhesus macaques againsr aerosolized ricin: Epitope-specific neutralizing antibodies correlated with protection. Proc. Natl. Acad. Sci. USA 2015, 112, 3782–3787. [Google Scholar] [PubMed]
- Smallshaw, J.E.; Vitetta, E.S. A lyophilized formulation of RiVax, a recombinant ricin subunit vaccine, retains immunogenicity. Vaccine 2010, 28, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, M.; Johnson, V.G.; Skopicki, H.A.; Fendley, B.; Lewis, M.S.; Youle, R.J. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J. Immunol. 1987, 138, 3339–3344. [Google Scholar] [PubMed]
- Maddaloni, M.; Cooke, C.; Wilkinson, R.; Stout, A.V.; Eng, L.; Pincus, S.H. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J. Immunol. 2004, 172, 6221–6228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Zhao, K.; Chen, J.; Feng, J.; Sun, Y.; Li, Y.; Shen, B. Novel chimeric anti-ricin antibody C4C13 with neutralizing activity against ricin toxicity. Biotechnol. Lett. 2007, 29, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Yermakova, A.; Mantis, N.J. Protective immunity to ricin toxin conferred by antibodies against the toxin′s binding subunit (RTB). Vaccine 2011, 29, 7925–7935. [Google Scholar] [CrossRef] [PubMed]
- O′Hara, J.M.; Yermakova, A.; Mantis, N.J. Immunity to ricin: Fundamental insights into toxin-antibody interactions. In Ricin and Shiga Toxins; Springer: Berlin/Heidelberg, Germany, 2011; Volume 357, pp. 209–241. [Google Scholar]
- Prigent, J.; Panigai, L.; Lamourette, P.; Sauvaire, D.; Devilliers, K.; Plaisance, M.; Vollnad, H.; Creminon, C.; Simon, S. Neutralising antibodies against ricin toxin. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Luo, L.; Wu, J.; Jia, P.; Li, Q.; Wang, Y.; Gao, Z.; Peng, H.; Lv, M.; Huang, C.; et al. Monoclonal antibody, mAb 4C13, an effective detoxicant antibody against ricin poisoning. Vaccine 2015, 33, 3836–3842. [Google Scholar] [CrossRef] [PubMed]
- O′Hara, J.; Whaley, K.; Pauly, M.; Zeitlin, L.; Mantis, N.J. Plant-based expression of a partially humanized neutralizing monoclonal IgG directed against an immunodominant epitope on the ricin toxin A subunit. Vaccine 2012, 30, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.H.; Das, A.; Song, K.; Maresh, G.A.; Corti, M.; Berry, J. Role of Fc in antibody-mediated protection from ricin toxin. Toxins 2014, 6, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.G.; Yin, J.; Chau, D.; Negrych, L.M.; Cherwonogrodzky, J.W. Humanization and characterization of an anti-ricin neutralization monoclonal antibody. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Mazor, O.; Alcalay, R.; Seliger, N.; Aftalion, M.; Sapoznikov, A.; Falach, R.; Kronman, C.; Sabo, T. Antibody/doxycycline combined therapy for pulmonary ricinosis: Attenuation of inflammation improves survival of ricin-intoxicated mice. Toxicol. Rep. 2014, 1, 496–504. [Google Scholar] [CrossRef]
- Cohen, O.; Mechaly, A.; Sabo, T.; Alcalay, R.; Aloni-Grinstein, R.; Seliger, N.; Kronman, C.; Mazor, O. Characterization and epitope mapping of the polyclonal antibody repertoire elicited by ricin-holotoxin based vaccination. Clin. Vaccine Immunol. 2014, 21, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Sabo, T.; Kronman, C.; Mazor, O. Ricin-holotoxin based vaccines: Induction of potent ricin neutralizing antibodies. Methods Mol. Biol. 2016, in press. [Google Scholar]
- Audette, G.F.; Vandonselaar, M.; Delbaere, T.J. The 2.2 Å resolution structure of the O(H) blood-group-specific lectin I from Ulex europaeus. J. Mol. Biol. 2000, 304, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.-P.; Dübel, S.; Thullier, P. Isolation of human like antibody fragment (ScFv) that neutralizes ricin biological activity. BMC Biotechnol. 2009, 9, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.; Mohseni, S.; Peipp, M. Mouse immune libraries for the generation of ScFv fragments directed against human cell surface antigens. In Antibody engineering; Kontermann, R., Dubel, S., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; pp. 47–63. [Google Scholar]
- Lefranc, M.; Giudicelli, V.; Ginetoux, C.; Jabado-Michaloud, J.; Folch, G.; Bellahcene, F.; Wu, Y.; Gemrot, E.; Brochet, X.; Lane, J.; et al. IMGT, the international ImMunoGeneTics information system®. Nucleic Acids Res. 2009, 37, D1006–D1012. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ma, N.; Madden, T.L.; Ostell, J.M. IgBLAST: An immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 2013, 41, W34–W40. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.; Alcalay, R.; Sabo, T.; Noy-Porat, T.; Epstein, E.; Kronman, C.; Mazor, O. Rapid assessment of antibody-induced ricin neutralization by employing a novel functional cell-based assay. J. Immunol. Methods 2015, 424, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Druar, C.; Saini, S.S.; Cossitt, M.A.; Yu, F.; Qiu, X.; Geisbert, T.W.; Jones, S.; Jahrling, P.B.; Stewart, D.I.; Weirsma, E.J. Analysis of the expressed heavy chain variable-region genes of Macaca fascicularis and isolation of monoclonal antibodies specific for the Ebola virus′ soluble glycoprotein. Immunogenetics 2005, 57, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Bible, J.M.; Howard, W.; Robbins, H.; Dunn-Walters, D.K. IGHV1, IGHV5 and IGHV7 subgroup genes in the rhesus macaque. Immunogenetics 2003, 54, 867–873. [Google Scholar] [PubMed]
- Margolin, D.H.; Reimann, K.A.; Sodroski, J.; Karlsson, G.B.; Tenner-Racz, K.; Letvin, N.L. Immunoglobulin V(H) usage during primary infection of rhesus minkeys with chimeric simian-human immunodeficiency viruses. J. Virol. 1997, 71, 8582–8591. [Google Scholar] [PubMed]
- Sundling, C.; Phad, G.; Douagi, L.; Navis, M.; Hedestam, G. Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J. Immunol. Methods 2012, 386, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Cohen, H.; Cohen, O.; Mazor, O. A biolayer interferometry-based assay for rapid and highly-sensitive detection of biowarfare agents. Biosens Bioelectron. 2016. submitted for publication. [Google Scholar]
- McGuinness, C.R.; Mantis, N.J. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect. Immun. 2006, 74, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- O′Hara, J.M.; Neal, L.M.; McCarthy, E.A.; Kasten-Jolly, J.A.; Brey, R.N.; Mantis, N.J. Folding domains within the ricin toxin a subunit as targets of protective antibodies. Vaccine 2010, 28, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Sully, E.; Whaley, K.; Bohorova, N.; Bohorov, O.; Goodman, C.; Kim, D.; Pauly, M.; Velasco, J.; Hiatt, E.; Morton, J.; et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin. Vaccine Immunol. 2014, 21, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Levy, H.; Epstein, E.; Rosenfeld, R.; Marcus, H.; Ben-Arie, E.; Shafferman, A.; Ordentlich, A.; Mazor, O. A novel mechanism for antibody-based anthrax toxin neutralization: Inhibition of prepore-to-pore conversion. J. Biol. Chem. 2012, 287, 32665–32673. [Google Scholar] [CrossRef] [PubMed]
- Noy-Porat, T.; Cohen, O.; Ehrlich, S.; Epstein, E.; Alcalay, R.; Mazor, O. Acetylcholinesterase-Fc fusion protein (AChE-Fc): A novel potential organophosphate bioscavenger with extended plasma half-life. Bioconjugate Chem. 2015, 26, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Neal, L.M.; O′Hara, J.; Brey, R.N.; Mantis, N.J. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect. Immun. 2010, 78, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.G.; Yin, J.; Chau, D.; Hu, C.C.; Lillico, D.; Yu, J.; Negrych, L.M.; Cherwonogrodzky, J.W. Conformation-dependent high-affinity potent ricin-neutralizing monoclonal antibodies. Biomed. Res. Int. 2013, 2013, 471346. [Google Scholar] [CrossRef] [PubMed]
- Azriel-Rosenfeld, R.; Valensi, M.; Benhar, I. A human synthetic combinatorial library of arrayable single-chain antibodies based on shuffling in vivo formed CDRs into general framework regions. J. Mol. Biol. 2004, 335, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Sabo, T.; Gal, Y.; Elhanany, E.; Sapoznikov, A.; Falach, R.; Mazor, O.; Kronman, C. Antibody treatment against pulmonary exposure to abrin confers significantly higher levels of protection that treatment against ricin intoxication. Toxicol. Lett. 2015, 237, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.; Marcus, H.; Ben-Arie, E.; Lachmi, B.; Mechaly, A.; Reuveny, S.; Gat, O.; Mazor, O.; Ordentlich, A. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against Lethal Bacillus anthracis infection. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Pica, C.M.; Song, J.; Luker, K.E.; Piwnica-Worms, D. Imaging 26S proteasome activity and inhibition in living mice. Nature Med. 2003, 9, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Sapoznikov, A.; Falach, R.; Mazor, O.; Alcalay, R.; Gal, Y.; Seliger, N.; Sabo, T.; Kronman, C. Diverse profiles of ricin-cell interactions in the lung following inranasal exposure to ricin. Toxins 2015, 7, 4817–4831. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Vaccine a | Binding b | ED50 (ng/mL) c | kon (1/Ms) | koff (1/s) | KD (nM) |
|---|---|---|---|---|---|---|
| MH1 | subunit | RTA | 200 d | 9 × 104 | <1 × 10−7 | <0.001 |
| MH2 | subunit | RTB | 5200 | 18 × 104 | 1 × 10−5 | 0.6 |
| MH36 | holotoxin | RTA | 27,000 | 11 × 104 | <1 × 10−7 | <0.001 |
| MH49 | holotoxin | RTA | 1200 | 7 × 104 | <1 × 10−7 | <0.001 |
| MH67 | holotoxin | RTA | 52,000 | 9 × 104 | 8 × 10−4 | 9.0 |
| MH73 | subunit | RTB | 11,000 | 11 × 104 | <1 × 10−7 | <0.001 |
| MH74 | subunit | RTA | 83,000 d | 15 × 104 | 7 × 10−3 | 45 |
| MH75 | subunit | RTB | 150 d | 35 × 104 | <1 × 10−7 | <0.001 |
| MH76 | subunit | RTB | 10,500 | 11 × 104 | 3 × 10−4 | 3.0 |
| MH77 | subunit | RTB | 500 | 17 × 104 | 1 × 10−7 | 0.001 |
| Antibody | VH | VL | |||
|---|---|---|---|---|---|
| V | D | J | V | J | |
| MH1 a | IGHV4.40 | IGHD2-4*01 | IGHJ1*01 | IGLV3.46 c | IGLJ3 |
| MH2 a | IGHV3.15 | IGHD5-3*01 | IGHJ5-1*01 | IGLV3.46 c | IGLJ2 |
| MH36 b | IGHV3.9 | IGHD4-2*01 | IGHJ4*01 | IGLV1.25 c | IGLJ2 |
| MH49 b | IGHV4.11 | IGHD4-2*01 | IGHJ3*01 | IGLV1.27 c | IGLJx1 |
| MH67 b | IGHV4.11 | IGHD4-2*01 | IGHJ3*01 | IGKV1.9 d | IGKJ2 |
| MH73 a | IGHV4.11 | IGHD1-2*01 | IGHJ5-1*01 | IGKV1.52 d | IGKJ2 |
| MH74 a | IGHV5.7 | IGHD3-3*01 | IGHJ5-2*02 | IGLV1.30 c | IGLJ3 |
| MH75 a | IGHV3.15 | IGHD5-3*01 | IGHJ5-1*01 | IGLV3.46 c | IGLJ2 |
| MH76 a | IGHV3.15 | IGHD3-1*01 | IGHJ4*01 | IGLV3.46 c | IGLJ3 |
| MH77 a | IGHV4.40 | IGHD4-4*01 | IGHJ5-1*01 | IGKV1.7 d | IGKJ2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noy-Porat, T.; Rosenfeld, R.; Ariel, N.; Epstein, E.; Alcalay, R.; Zvi, A.; Kronman, C.; Ordentlich, A.; Mazor, O. Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates. Toxins 2016, 8, 64. https://doi.org/10.3390/toxins8030064
Noy-Porat T, Rosenfeld R, Ariel N, Epstein E, Alcalay R, Zvi A, Kronman C, Ordentlich A, Mazor O. Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates. Toxins. 2016; 8(3):64. https://doi.org/10.3390/toxins8030064
Chicago/Turabian StyleNoy-Porat, Tal, Ronit Rosenfeld, Naomi Ariel, Eyal Epstein, Ron Alcalay, Anat Zvi, Chanoch Kronman, Arie Ordentlich, and Ohad Mazor. 2016. "Isolation of Anti-Ricin Protective Antibodies Exhibiting High Affinity from Immunized Non-Human Primates" Toxins 8, no. 3: 64. https://doi.org/10.3390/toxins8030064