Structure-Activity Relationship of Chlorotoxin-Like Peptides
Abstract
:1. Introduction
2. Results
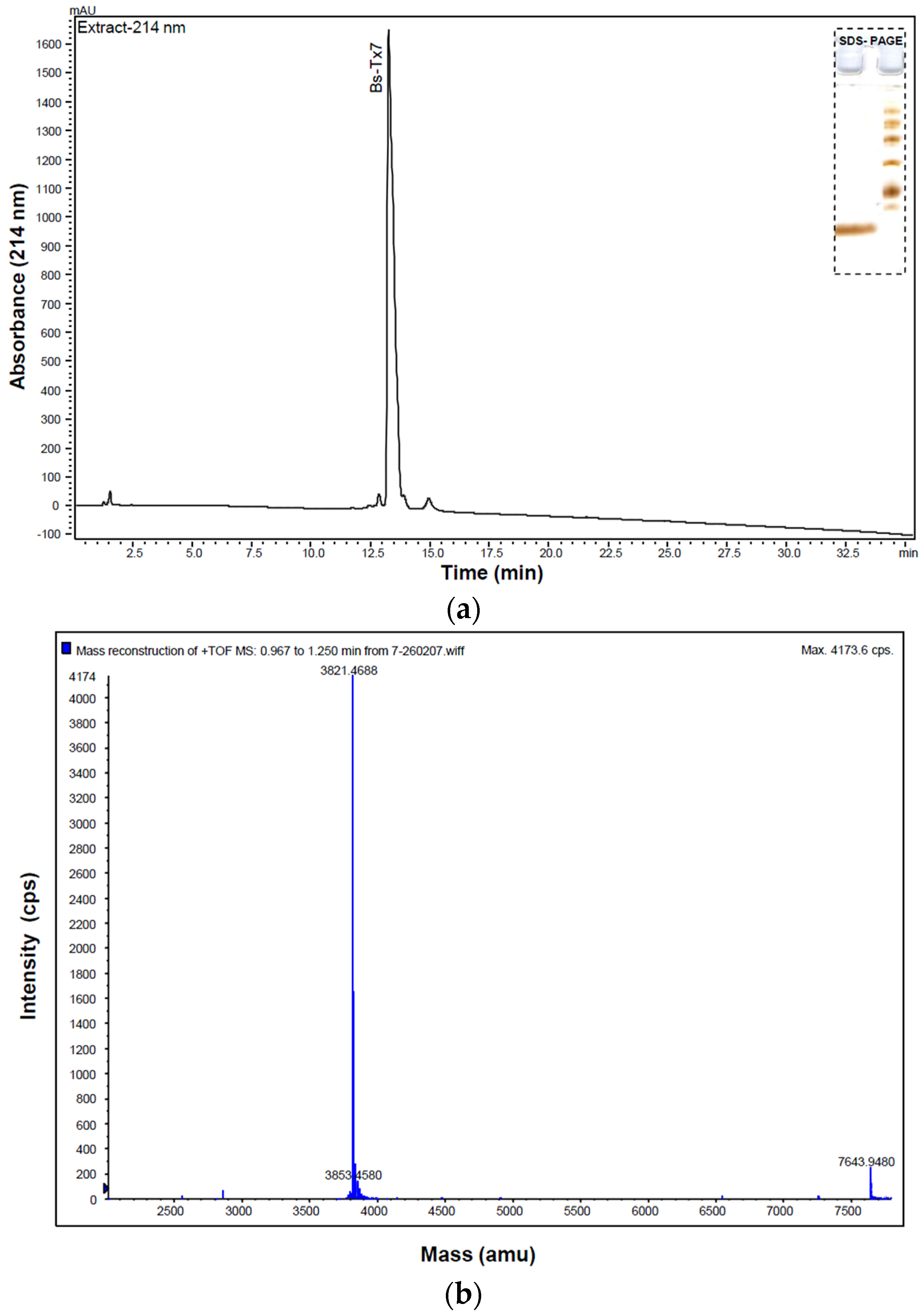
2.1. Purification and Structural Elucidation of Peptide Bs-Tx7

| Peptide | Residue Number | Sequence * | Mass Observed | Mass Calculated | Missed Cleavage |
|---|---|---|---|---|---|
| Tryptic cleavage products-Oxidized: | |||||
| T1 | 1–14 | CGPCFTTDWESEKK | 1741.8307 | 1741.8578 | 1 |
| T2 | 15–24 | CAECCGGIGR | 1112.1258 | 1113.1495 | - |
| T3 | 25–35 | CFGPQCLCNRK | 1413.6722 | 1413.556 | 1 |
| Non specific cleavage products: | |||||
| T3a | 25–29 | CFGPQ | 599.2600 | 599.17 | 0 |
| T3b | 30–35 | CLCNRK(2K+, H+) | 816.3808 | 814.116 | 0 |
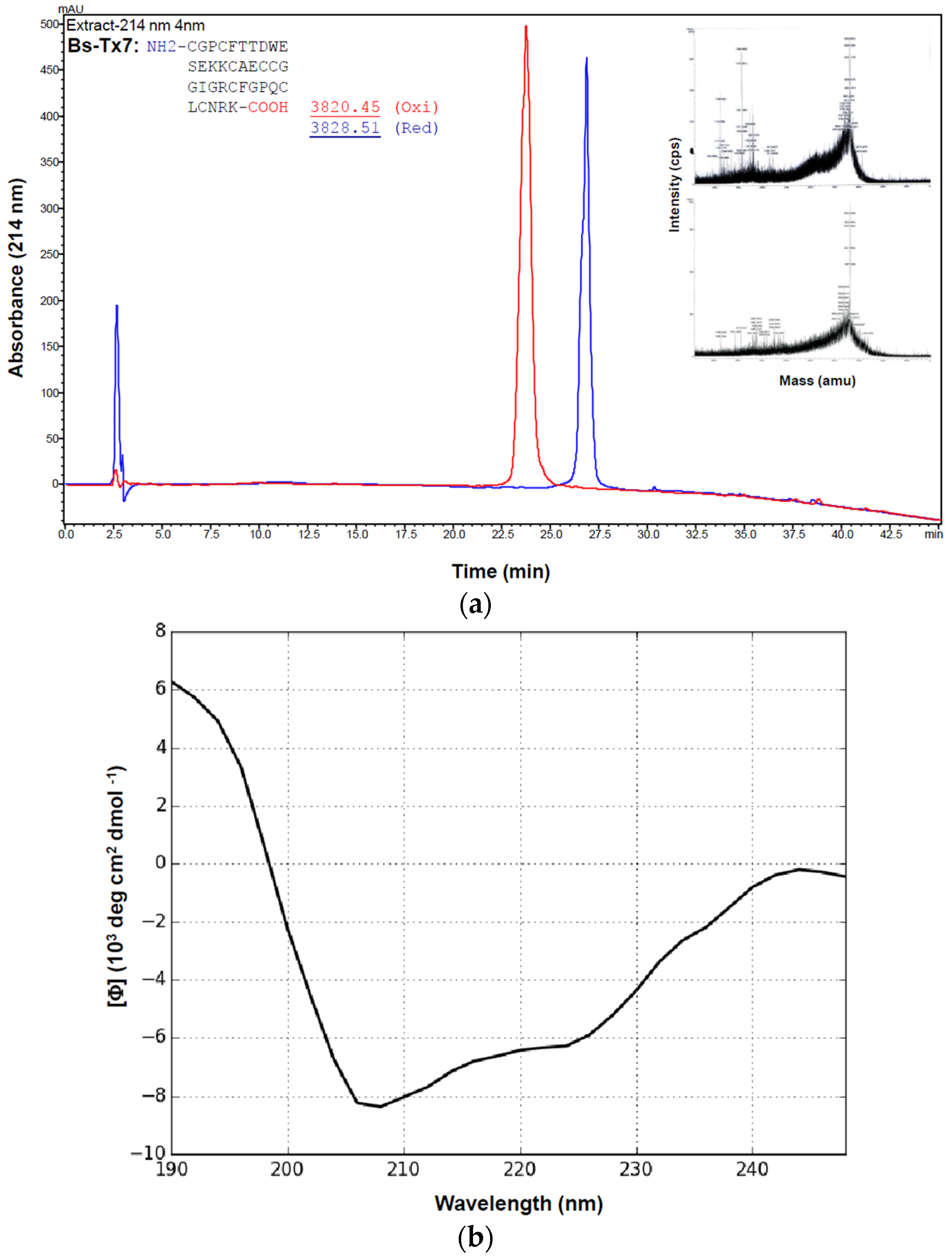
2.2. Synthesis and Folding of Bs-Tx7
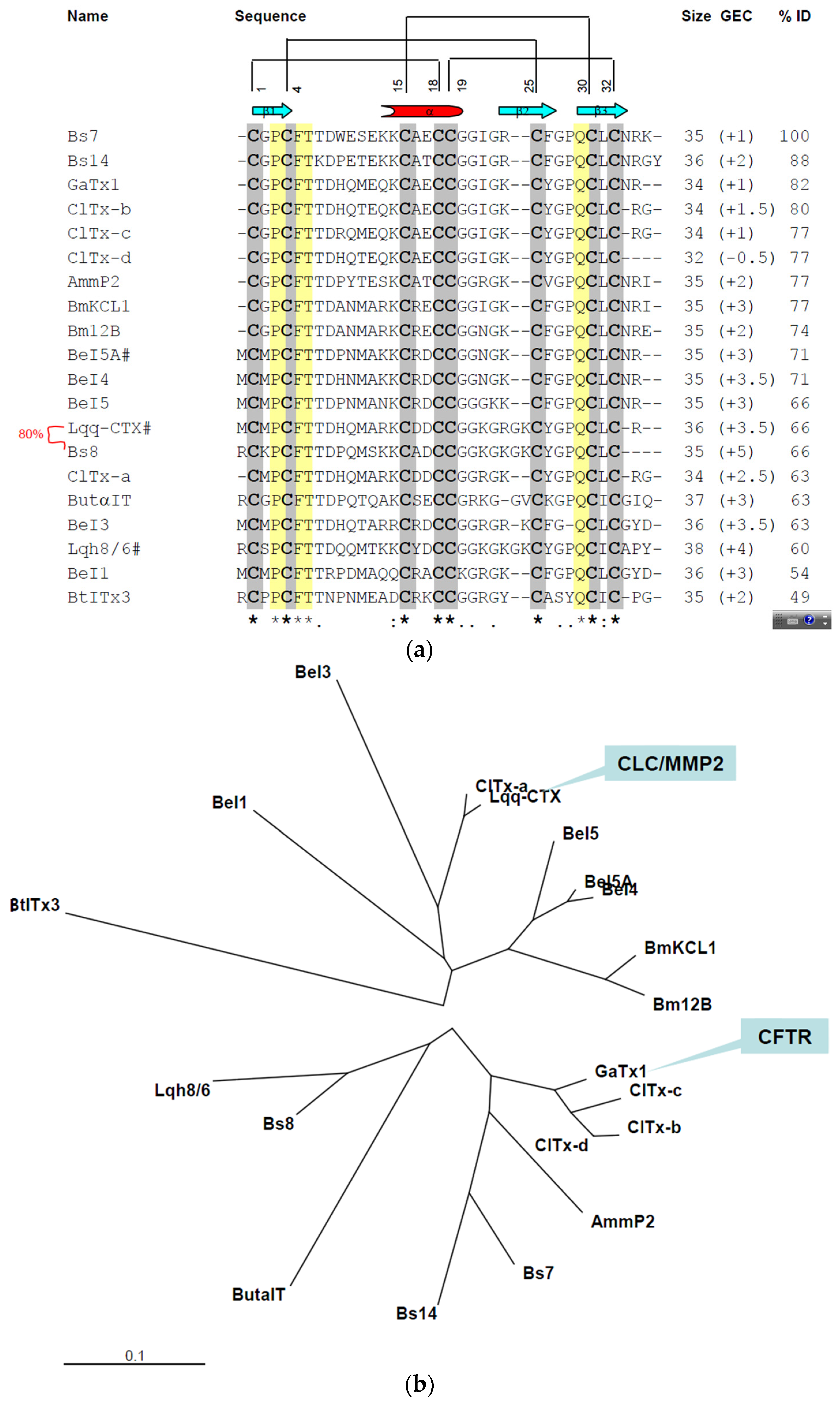
2.3. In Silico Studies on Bs-Tx7

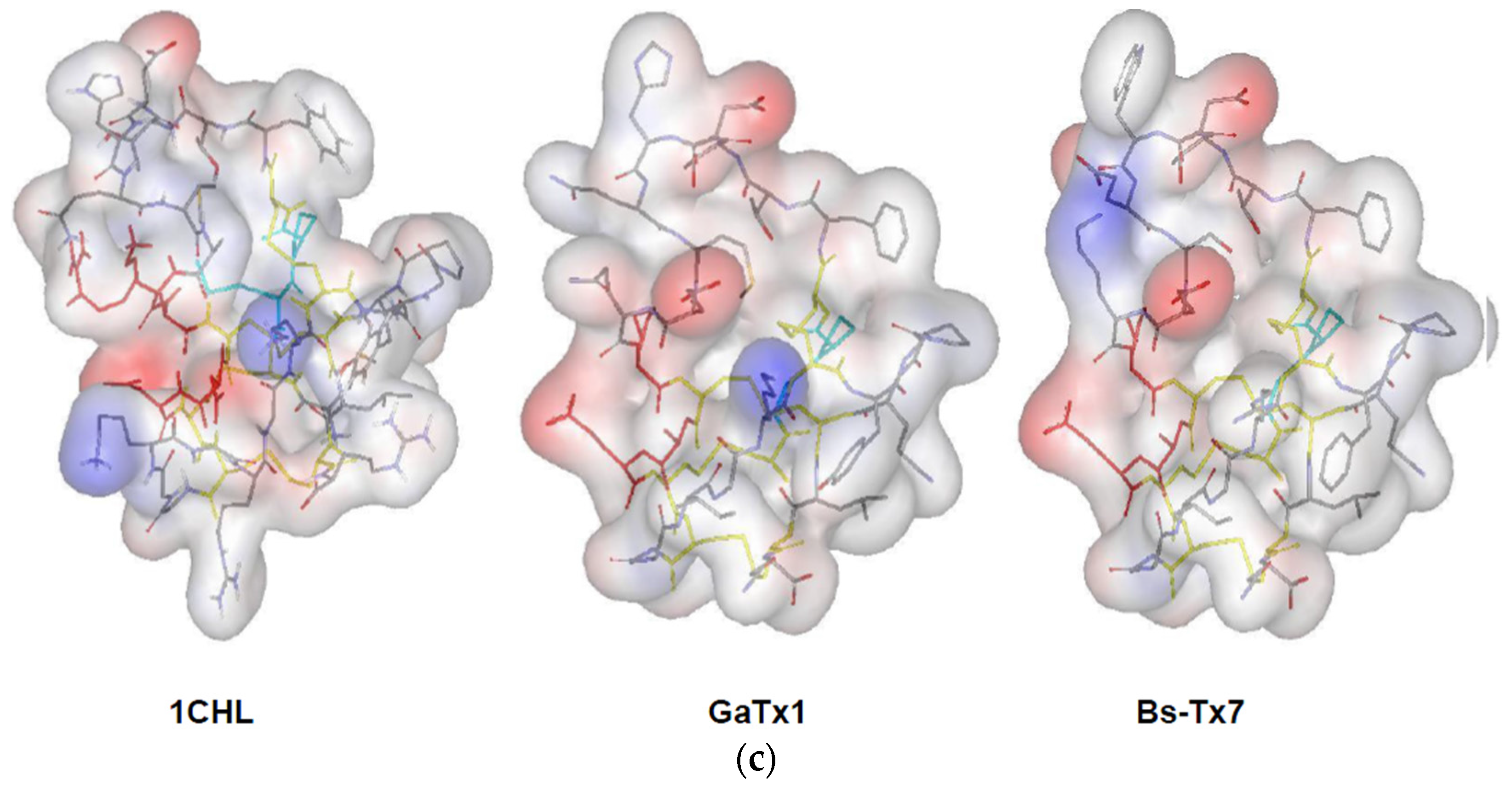
2.4. MMP2 Activation by Bs-Tx7


3. Discussion
4. Experimental Section
4.1. Isolation, Purification and Characterization
4.2. Primary Structure Elucidation of Bs-Tx7
4.3. Solid Phase Peptide Synthesis of Bs-Tx7
4.4. Continuous Fluorescence MMP2 Assay
4.5. In Silico Studies
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Tropica 2008, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Merino, E.; Corona, M.; Bolivar, F.; Becerril, B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 2000, 82, 861–868. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Goudet, C.; Chi, C.W.; Tytgat, J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 2002, 40, 1239–1258. [Google Scholar] [CrossRef]
- Tytgat, J.; Chandy, K.G.; Garcia, M.L.; Gutman, G.A.; Martin-Eauclaire, M.-F.; van der Walt, J.J.; Possani, L.D. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999, 20, 444–447. [Google Scholar] [CrossRef]
- Valdivia, H.H.; Kirby, M.S.; Lederer, W.J.; Coronado, R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca+2-release channel of skeletal and cardiac muscle. Proc. Natl. Acad. Sci. USA 1992, 89, 12185–12189. [Google Scholar] [CrossRef] [PubMed]
- Martin-Eauclaire, M.F.; Ceard, B.; Bosmans, F.; Rosso, J.P.; Tytgat, J.; Bougis, P.E. New Birtoxin analogs from Androctonus australis venom. Biochem. Biophys. Res. Commun. 2005, 333, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gao, B. Molecular characterization of a new scorpion venom lipolysis activating peptide: Evidence for disulfide bridge-mediated functional switch of peptides. FEBS Lett. 2006, 580, 6825–6836. [Google Scholar] [CrossRef] [PubMed]
- Gilles, N.; Blanchet, C.; Shichor, I.; Zaninetti, M.; Lotan, I.; Bertrand, D.; Gordon, D. A scorpion alpha-like toxin that is active on insects and mammals reveals an unexpected specificity and distribution of sodium channel subtypes in rat brain neurons. J. Neurosci. 1999, 19, 8730–8739. [Google Scholar] [PubMed]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993, 264, 361–369. [Google Scholar]
- Rosso, J.P.; Rochat, H. Characterization of ten proteins from the venom of the Moroccan scorpion Androctonus mauretanicus mauretanicus, six of which are toxic to the mouse. Toxicon 1985, 23, 113–125. [Google Scholar] [CrossRef]
- Ali, S.A.; Stoeva, S.; Schütz, J.; Kayed, R.; Abbasi, A.; Zaidi, Z.H.; Voelter, W. Purification and primary structure of low molecular mass peptides from scorpion (Buthus sindicus) venom. Comp. Biochem. Physiol. A 1998, 121, 323–332. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; de Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [PubMed]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shi, X.; Zhao, J. Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma. Curr Top Med Chem. 2015, 15, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, M.; Gabikian, P.; Bahrami, S.B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Stoeva, S.; Grossmann, J.G.; Abbasi, A.; Voelter, W. Purification, characterization, and primary structure of four depressant insect-selective neurotoxin analogs from scorpion (Buthus sindicus) venom. Arch. Biochem. Biophys. 2001, 391, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Yang, D.; Jackson, T.N.W.; Undheim, E.A.B.; Koludarov, I.; Wood, K.; Jones, A.; Hodgson, W.C.; McCarthy, S.; Ruder, T.; et al. Venom proteomic characterization and relative antivenom neutralization of two medically important Pakistani Elapid snakes (Bungarus sindanus and Najanaja). J Proteomics 2013, 89, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.A.; Abbasi, A.; Kalbacher, H.; Voelter, W. Design and synthesis of a peptidyl-FRET substrate for tumor marker enzyme human matrix metalloprotease-2 (hMMP-2). Int. J. Pept. Res. Ther. 2012, 18, 207–215. [Google Scholar] [CrossRef]
- Ali, S.A.; Alam, M.; Abbasi, A.; Kalbacher, H.; Schaechinger, T.J.; Hu, Y.; Zhijian, C.; Li, W.; Voelter, W. Structure–activity relationship of a highly selective peptidyl inhibitor of Kv1.3 voltage-gated K+-channel from Scorpion (B. sindicus) venom. Int. J. Pept. Res. Ther. 2014, 20, 19–32. [Google Scholar] [CrossRef]
- Kohl, B.; Rothenberg, I.; Ali, S.A.; Alam, M.; Seebohm, G.; Kalbacher, H.; Voelter, W.; Stoll, R. Solid phase synthesis, NMR structure determination of α-KTx3.8, its in silico docking to Kv1.x potassium channels, and electrophysiological analysis provide insights into toxin-channel selectivity. Toxicon 2015, 101, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A.; Boeckmann, B. The SWISS-PROT protein sequence data bank. Nucleic Acids Res. 1991, 19, 2247–2249. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R. Comparison of methods for searching protein sequence databases. Protein Sci. 1995, 4, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP: Phylogeny Inference Package; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Šali, A.; Blundell, T.L. Definition of general topological equivalence in protein structures: A procedure involving comparison of properties and relationships through simulated annealing and dynamic programming. J. Mol. Biol. 1990, 2, 403–428. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez-López, C.E.; Francke, O.F.; Ureta, C.; Possani, L.D. Scorpions from Mexico: From species diversity to venom complexity. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Wang, B.; Alam, M.; Beck, A.; Stoeva, S.; Voelter, W.; Abbasi, A.; Duszenko, M. Structure-activity relationship of an α-toxin Bs-Tx28 from scorpion (Buthus sindicus) venom suggests a new α-toxin subfamily. Arch. Biochem. Biophys. 2006, 445, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Strasberg-Rieber, M.; Rieber, M. Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin. Exp. Metastasis 2005, 22, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Günther, W. Chloride channels: An emerging molecular picture. BioEssays 2005, 19, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar] [PubMed]
- Maertens, C.; Wei, L.; Tytgat, J.G.; Droogmans, B.; Nilius, B. Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels. Br. J. Pharmacol. 2000, 129, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- McFerrin, M.B.; Sontheimer, H. A role for ion channels in glioma cell invasion. Neuron Glia. Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Sun, Z.; Jiang, D.; Dai, C.; Ma, Y.; Zhao, Z.; Liu, H.; Wu, Y.; Cao, Z.; Li, W. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010, 291, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Yin, L.T.; Liang, A.H.; Zhang, C.F.; Wang, W.; Chai, B.F.; Yang, J.Y.; Fan, X.J. Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci Lett. 2007, 412, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.D.; Thompson, C.H.; Zhang, Z.R.; Freeman, C.S.; Schay, E.; Szakács, G.; Szakács, G.; Bakos, E.; Sarkadi, B.; McMaster, D.; et al. State-dependent inhibition of cystic fibrosis transmembrane conductance regulator chloride channels by a novel peptide toxin. J. Biol. Chem. 2007, 282, 37545–37555. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.; Zhang, M. Inhibition of tumor-cell invasion with chlorotoxin-bound super paramagnetic nanoparticles. Small 2009, 5, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100,000 by laser ionization time-of flight mass spectrometry. Rapid Commun Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Strupat, K.; Karas, M.; Hillenkamp, F. 2,5-Dihidroxybenzoic acid: A new matrix for laser desorption-ionization mass spectrometry. Int. J. Mass Spectrom. Ion Processes 1991, 72, 89–102. [Google Scholar] [CrossRef]
- Sanger, F. Fractionation of oxidized insulin. Biochem. J. 1949, 44, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Hirs, C.H.W. Enzyme Structure, Methods in Enzymology; Academic Press: New York, NY, USA, 1967; Volume XI. [Google Scholar]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F. Electrospray ionization—Principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Hewick, R.M.; Hunkapillar, M.W.; Hood, L.E.; Dreyer, W.J. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 1981, 256, 7990–7997. [Google Scholar] [PubMed]
- Edman, P.; Begg, G. A protein sequenator. Eur. J. Biochem. 1967, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Lützner, N.; Pätzold, B.; Zoll, S.; Stehle, T.; Kalbacher, H. Development of a novel fluorescent substrate for Autolysin E, a bacterial type II amidase. Biochem. Biophys. Res. Commun. 2009, 380, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Adjadj, E.; Naudat, V.; Quiniou, E.; Wouters, D.; Sautiére, P.; Craescu, C.T. Solution structure of Lqh-8:6, a toxin-like peptide from scorpion venom. Eur. J. Biochem. 1997, 246, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Swiss-Modeling Server. Available online: http://swissmodel.expasy.org (accessed on 28 January 2016).
- Laskowski, R.A.; McArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK—A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Vriend, G. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 1990, 29, 52–56. [Google Scholar] [CrossRef]
- Lim, N.K.; Villemagne, V.L.; Soon, C.P.; Laughton, K.M.; Rowe, C.C.; McLean, C.A.; Masters, C.L.; Evin, G.; Li, Q.X. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer's disease. J Alzheimers Dis. 2011, 26, 779–786. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.A.; Alam, M.; Abbasi, A.; Undheim, E.A.B.; Fry, B.G.; Kalbacher, H.; Voelter, W. Structure-Activity Relationship of Chlorotoxin-Like Peptides. Toxins 2016, 8, 36. https://doi.org/10.3390/toxins8020036
Ali SA, Alam M, Abbasi A, Undheim EAB, Fry BG, Kalbacher H, Voelter W. Structure-Activity Relationship of Chlorotoxin-Like Peptides. Toxins. 2016; 8(2):36. https://doi.org/10.3390/toxins8020036
Chicago/Turabian StyleAli, Syed Abid, Mehtab Alam, Atiya Abbasi, Eivind A. B. Undheim, Bryan Grieg Fry, Hubert Kalbacher, and Wolfgang Voelter. 2016. "Structure-Activity Relationship of Chlorotoxin-Like Peptides" Toxins 8, no. 2: 36. https://doi.org/10.3390/toxins8020036