Transcript Abundance of Photorhabdus Insect-Related (Pir) Toxin in Manduca sexta and Galleria mellonella Infections
Abstract
:1. Introduction
2. Results
2.1. PirAB plu4093-2 Transcript Expression in Natural Infections
2.2. Portal of Entry and Time Until Death in Forced Infections
2.3. Transcript Abundance in Forced Infection Assays
3. Discussion
4. Experimental Section
4.1. Insects, Nematodes, and Bacterial Cultures
4.2. Natural and Forced Infection Assays
4.3. Detection of pirAB Transcripts through qPCR
4.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaugler, R.; Kaya, H.K. Entomopathogenic Nematodes in Biological Control; CRC Press, Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Gaugler, R.; Gaugler, R. Entomopathogenic Nematology; CABI Pub.: Cambridge, MA, USA, 2002. [Google Scholar]
- Koppenhoefer, A.M.; Lacey, L.A.; Kaya, H.K. Nematodes; Kluwer Academic Publishers: Berlin, Germany, 2000. [Google Scholar]
- Thomas, G.M.; Poinar, G.O. Xenorhabdus Gen-Nov, A Genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 1979, 29, 352–360. [Google Scholar] [CrossRef]
- Boemare, N.E.; Akhurst, R.J.; Mourant, R.G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new Genus, Photorhabdus gen. nov. Int. J. Syst. Bacteriol. 1993, 43, 249–255. [Google Scholar] [CrossRef]
- Bedding, R.A.; Molyneux, A.S. Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae, Nematoda). Nematologica 1982, 28, 354–359. [Google Scholar] [CrossRef]
- Grewal, P.S.; Gaugler, R.; Lewis, E.E. Host recognition behavior by entomopathogenic nematodes during contact with insect gut contents. J. Parasitol. 1993, 79, 495–503. [Google Scholar] [CrossRef]
- Wang, Y.; Gaugler, R. Host and penetration site location by entomopathogenic nematodes against Japanese beetle larvae. J. Invertebr. Pathol. 1998, 72, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Waterfield, N.R.; Daborn, P.J.; Dean, P.; Chilver, T.; Au, C.P.Y.; Sharma, S.; Potter, U.; Reynolds, S.E.; ffrench-Constant, R.H. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell. Microbiol. 2002, 4, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Duchaud, E.; Rusniok, C.; Frangeul, L.; Buchrieser, C.; Givaudan, A.; Taourit, S.; Bocs, S.; Boursaux-Eude, C.; Chandler, M.; Charles, J.F.; et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 2003, 21, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.; Hares, M.; Yang, G.; Dowling, A.; ffrench-Constant, R. Potentiation and cellular phenotypes of the insecticidal Toxin complexes of Photorhabdus bacteria. Cell. Microbiol. 2005, 7, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Daborn, P.J.; Waterfield, N.; Silva, C.P.; Au, C.P.Y.; Sharma, S.; Ffrench-Constant, R.H. A single Photorhabdus gene, makes caterpillars floppy (MCF), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. USA 2002, 99, 10742–10747. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.J.; Daborn, P.J.; Waterfield, N.R.; Wang, P.; Streuli, C.H.; ffrench-Constant, R.H. The insecticidal toxin Makes caterpillars floppy (MCF) promotes apoptosis in mammalian cells. Cell. Microbiol. 2004, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Dowling, A.J.; Gerike, U.; ffrench-Constant, R.H.; Waterfield, N.R. Photorhabdus virulence cassettes confer injectable insecticidal activity against the wax moth. J. Bacteriol. 2006, 188, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, M.B.; Farrar, R.R.; Novak, N.G.; Lawrence, S.D. Remarkable susceptibility of the diamondback moth (Plutella xylostella) to ingestion of Pir toxins from Photorhabdus luminescens. Entomol. Exp. Appl. 2006, 121, 31–37. [Google Scholar] [CrossRef]
- Waterfield, N.; Kamita, S.G.; Hammock, B.D.; Ffrench-Constant, R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol. Lett. 2005, 245, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, C.; Qiu, L. Cloning, expression and insecticidal activity of the pirA2B2 gene from Photorhabdus luminescens TT01. Weishengwu Xuebao 2012, 52, 532–537. [Google Scholar]
- Ahantarig, A.; Chantawat, N.; Waterfield, N.R.; Ffrench-Constant, R.; Kittayapong, P. PirAB toxin from Photorhabdus asymbiotica as a larvicide against dengue vectors. Appl. Environ. Microbiol. 2009, 75, 4627–4629. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, X.; Zhang, X.; Liu, Z.; Ding, X.; Xia, L.; Hu, S. Photorhabdus luminescens PirAB-fusion protein exhibits both cytotoxicity and insecticidal activity. FEMS Microbiol. Lett. 2014, 356, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Chen, I.T.; Yang, Y.-T.; Ko, T.-P.; Huang, Y.-T.; Huang, J.-Y.; Huang, M.-F.; Lin, S.-J.; Chen, C.-Y.; Lin, S.-S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, R.H.; Dowling, A.; Waterfield, N.R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 2007, 49, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Z.; Li, Y.; Ding, X.; Xia, L.; Hu, S. PirB-Cry2Aa hybrid protein exhibits enhanced insecticidal activity against Spodoptera exigua larvae. J. Invertebr. Pathol. 2014, 120, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Muench, A.; Stingl, L.; Jung, K.; Heermann, R. Photorhabdus luminescens genes induced upon insect infection. MCB Genom. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Marokhazi, J.; Millichap, P.J.; Hodgkinson, A.J.; Sriboonlert, A.; ffrench-Constant, R.H.; Reynolds, S.E. Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: Roles of immune-related proteins shown by RNA interference. Insect Biochem. Mol. Biol. 2006, 36, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Daborn, P.J.; Waterfield, N.; Blight, M.A.; Ffrench-Constant, R.H. Measuring virulence factor expression by the pathogenic bacterium Photorhabdus luminescens in culture and during insect infection. J. Bacteriol. 2001, 183, 5834–5839. [Google Scholar] [CrossRef] [PubMed]
- Miranda, V.A.; Navarro, P.D.; Davidowitz, G.; Bronstein, J.; Stock, S.P. Effect of insect host age and diet on the fitness of the entomopathogenic nematode-bacteria mutualism. Symbiosis 2013, 61, 145–153. [Google Scholar] [CrossRef]
- Stock, S.P.; Goodrich-Blair, H.E. Nematode parasites, pathogens and associates of insects and invertebrates of economic importance. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Elsevier Ltd.: San Diego, CA, USA, 2012; pp. 373–426. [Google Scholar]

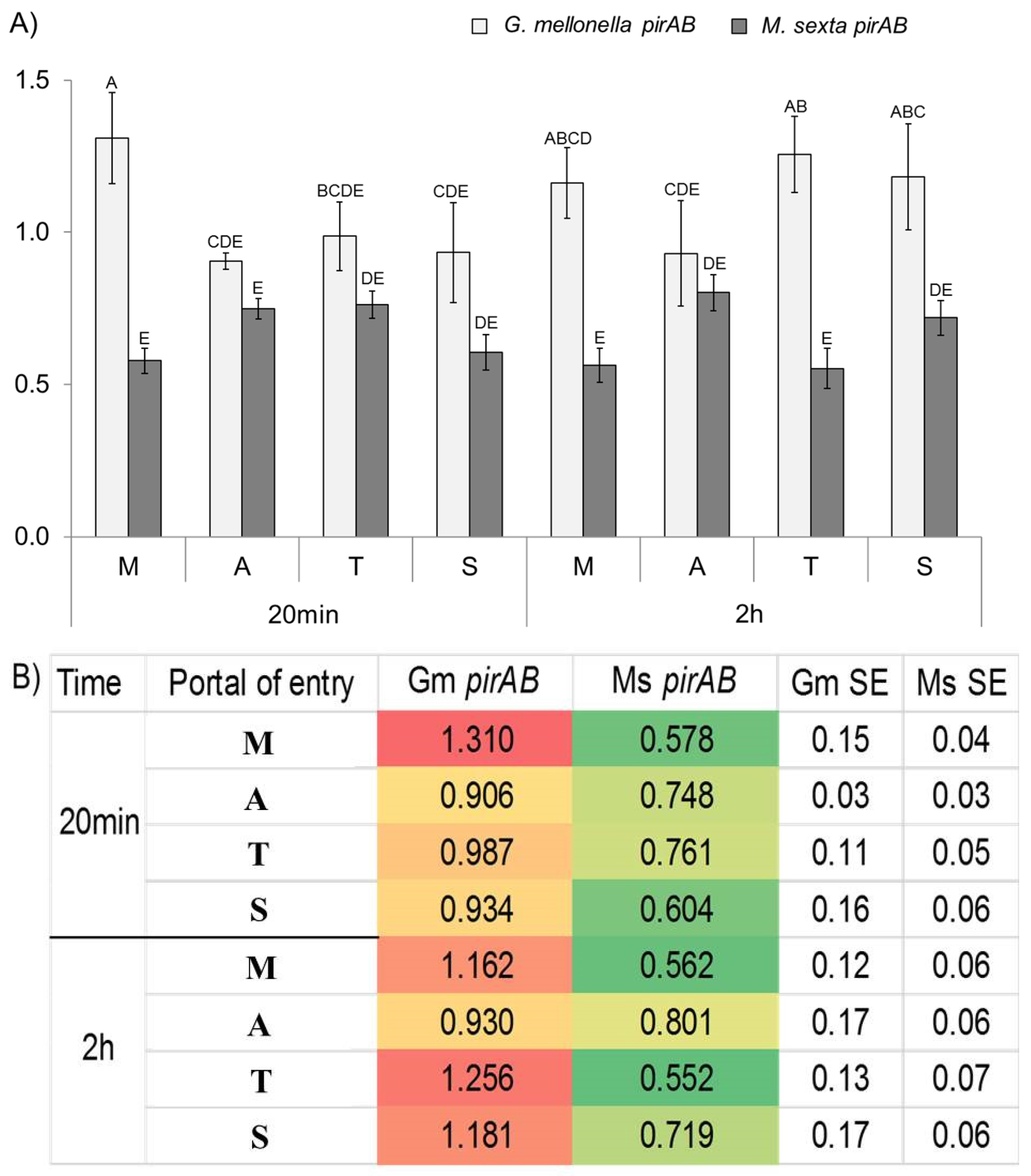
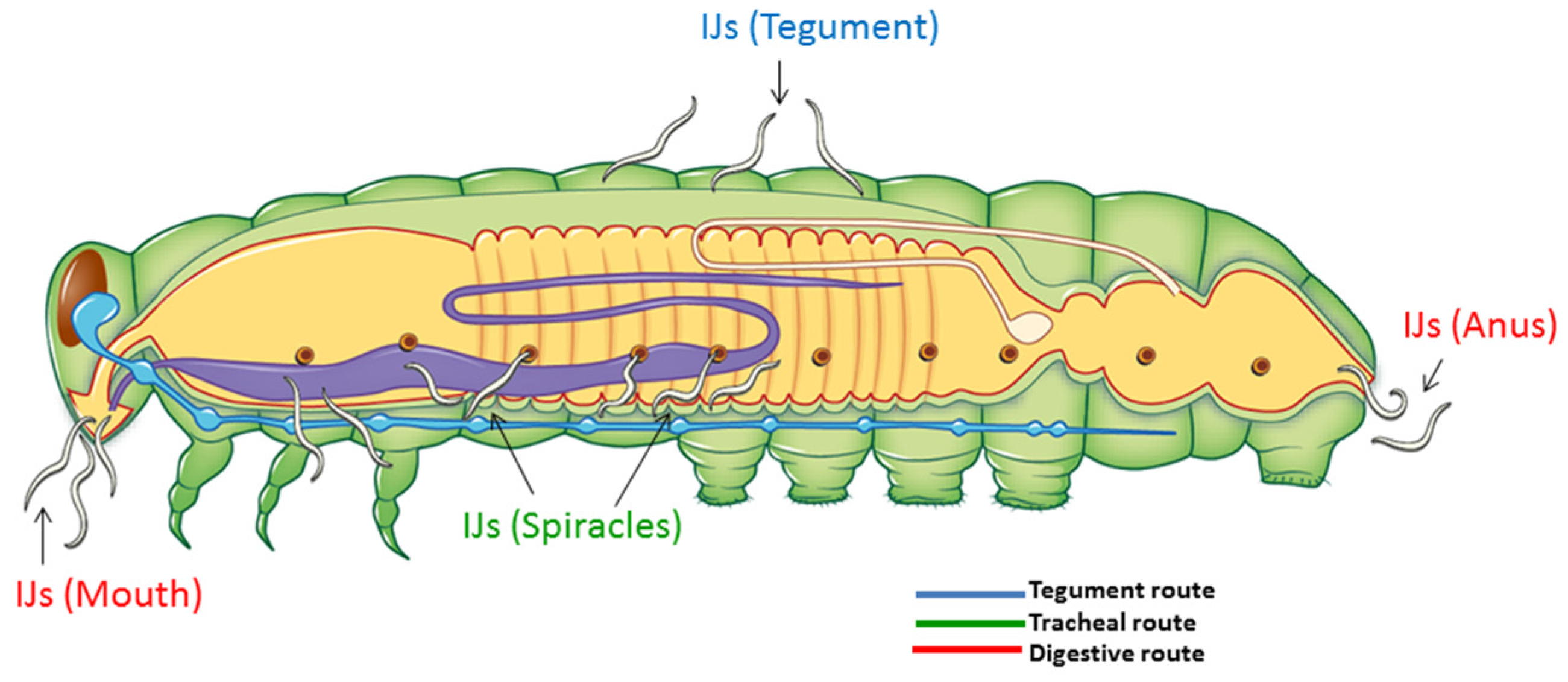
| Portal of Entry | Time Until Death (h ± SE) | ||
|---|---|---|---|
| G. mellonella * | M. sexta * | ||
| Mouth | 21 a ± 0.72 | 33 a ± 2.47 | |
| Anus | 24 b ± 0.85 | 28 a,b ± 2.31 | |
| Spiracle | 23 a,b ± 1.21 | 26 b ± 1.42 | |
| Tegument | 22 a,b ± 0.74 | 25 b ± 1.65 | |
| Toxin Transcript Detected | Gene ID | Amplicon Size (bp) | Sequence | Tm (°C) | GC (%) |
|---|---|---|---|---|---|
| pirAB | plu4093-2 | 231 | GGCACGTTAACACCTTCTCT | 58 | 48 |
| TGCAGTTGGTCCTTTGAGTGA | 62 | 48 | |||
| gyrB | plu004 | 105 | CTCGTGAAGCAGCCCGTAAA | 64 | 55 |
| CCGGATCACGTTCCTGACAA | 64 | 55 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagnola, A.; Mulley, G.; Davis, N.; Waterfield, N.; Stock, S.P. Transcript Abundance of Photorhabdus Insect-Related (Pir) Toxin in Manduca sexta and Galleria mellonella Infections. Toxins 2016, 8, 287. https://doi.org/10.3390/toxins8100287
Castagnola A, Mulley G, Davis N, Waterfield N, Stock SP. Transcript Abundance of Photorhabdus Insect-Related (Pir) Toxin in Manduca sexta and Galleria mellonella Infections. Toxins. 2016; 8(10):287. https://doi.org/10.3390/toxins8100287
Chicago/Turabian StyleCastagnola, Anaïs, Geraldine Mulley, Nathaniel Davis, Nicholas Waterfield, and S. Patricia Stock. 2016. "Transcript Abundance of Photorhabdus Insect-Related (Pir) Toxin in Manduca sexta and Galleria mellonella Infections" Toxins 8, no. 10: 287. https://doi.org/10.3390/toxins8100287
APA StyleCastagnola, A., Mulley, G., Davis, N., Waterfield, N., & Stock, S. P. (2016). Transcript Abundance of Photorhabdus Insect-Related (Pir) Toxin in Manduca sexta and Galleria mellonella Infections. Toxins, 8(10), 287. https://doi.org/10.3390/toxins8100287






