A Rapid LC-HRMS Method for the Determination of Domoic Acid in Urine Using a Self-Assembly Pipette Tip Solid-Phase Extraction
Abstract
:1. Introduction
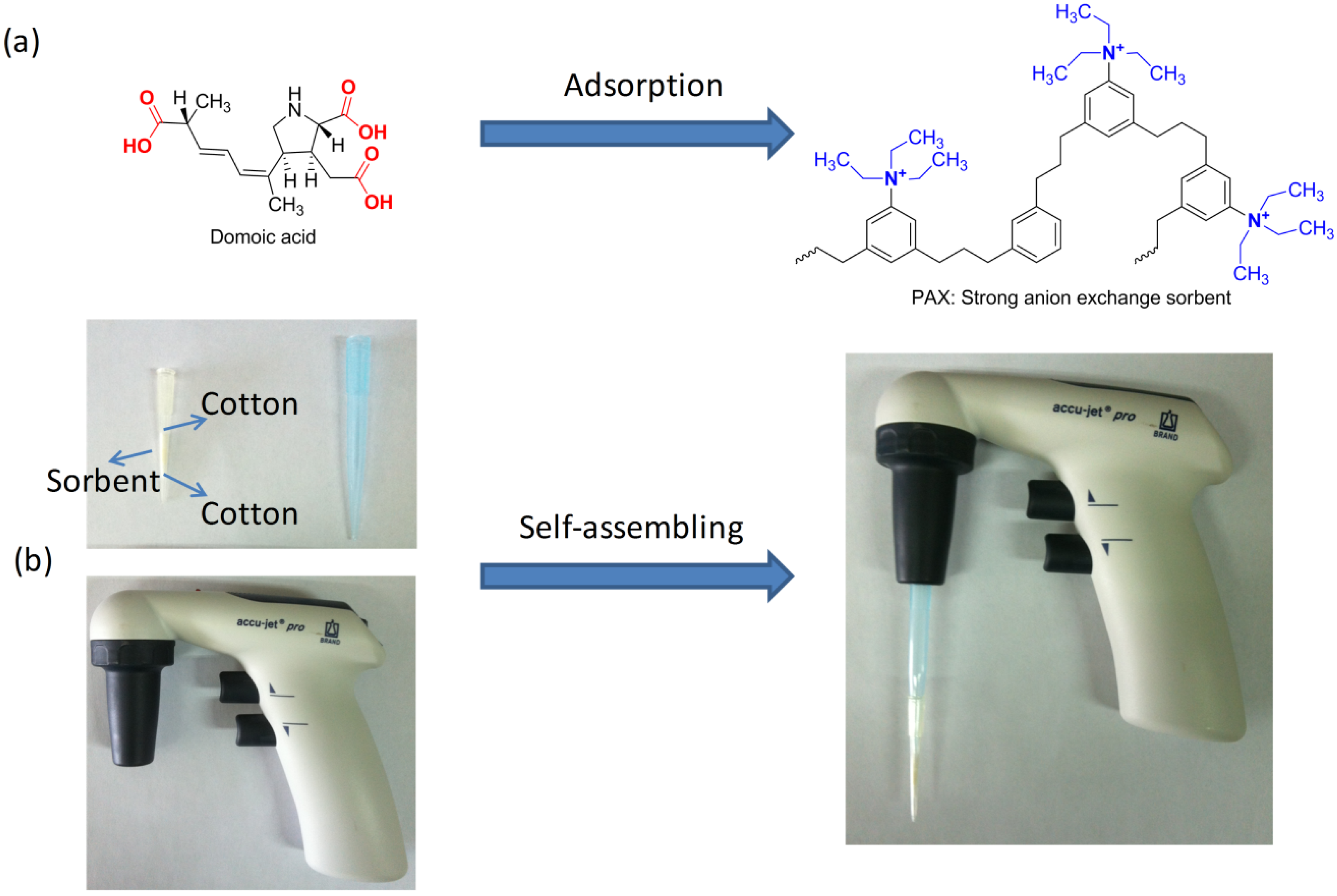
2. Results and Discussion
2.1. Separation of Domoic Acid
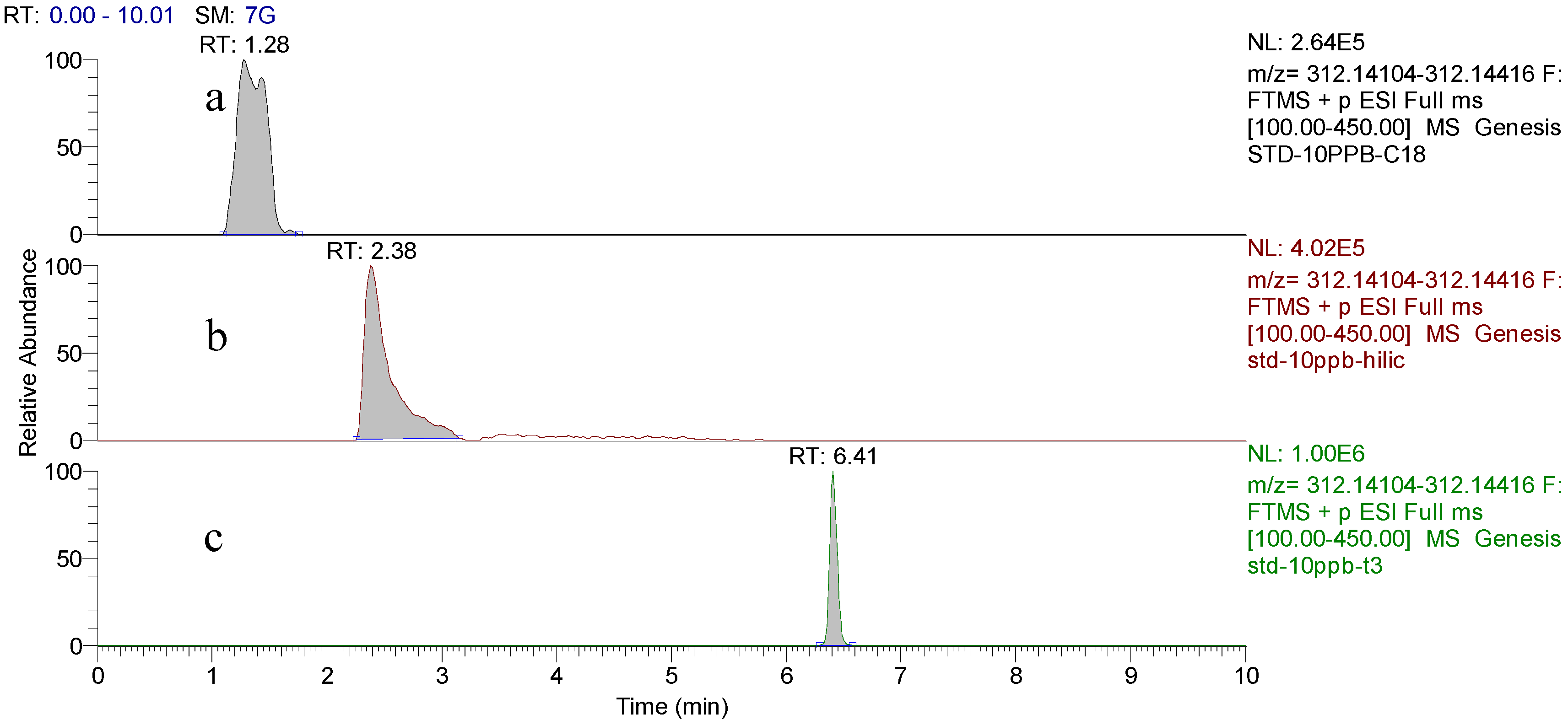
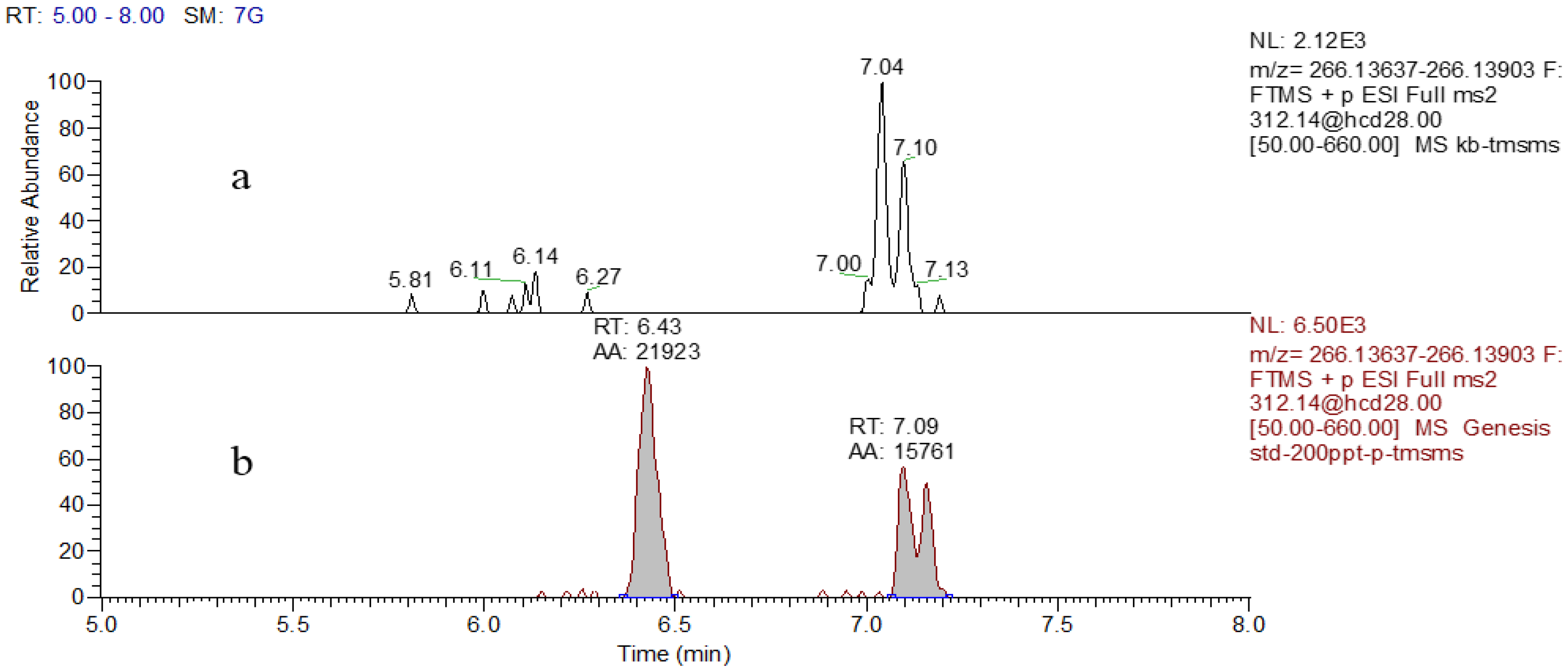
2.2. HR-MS/MS Analysis

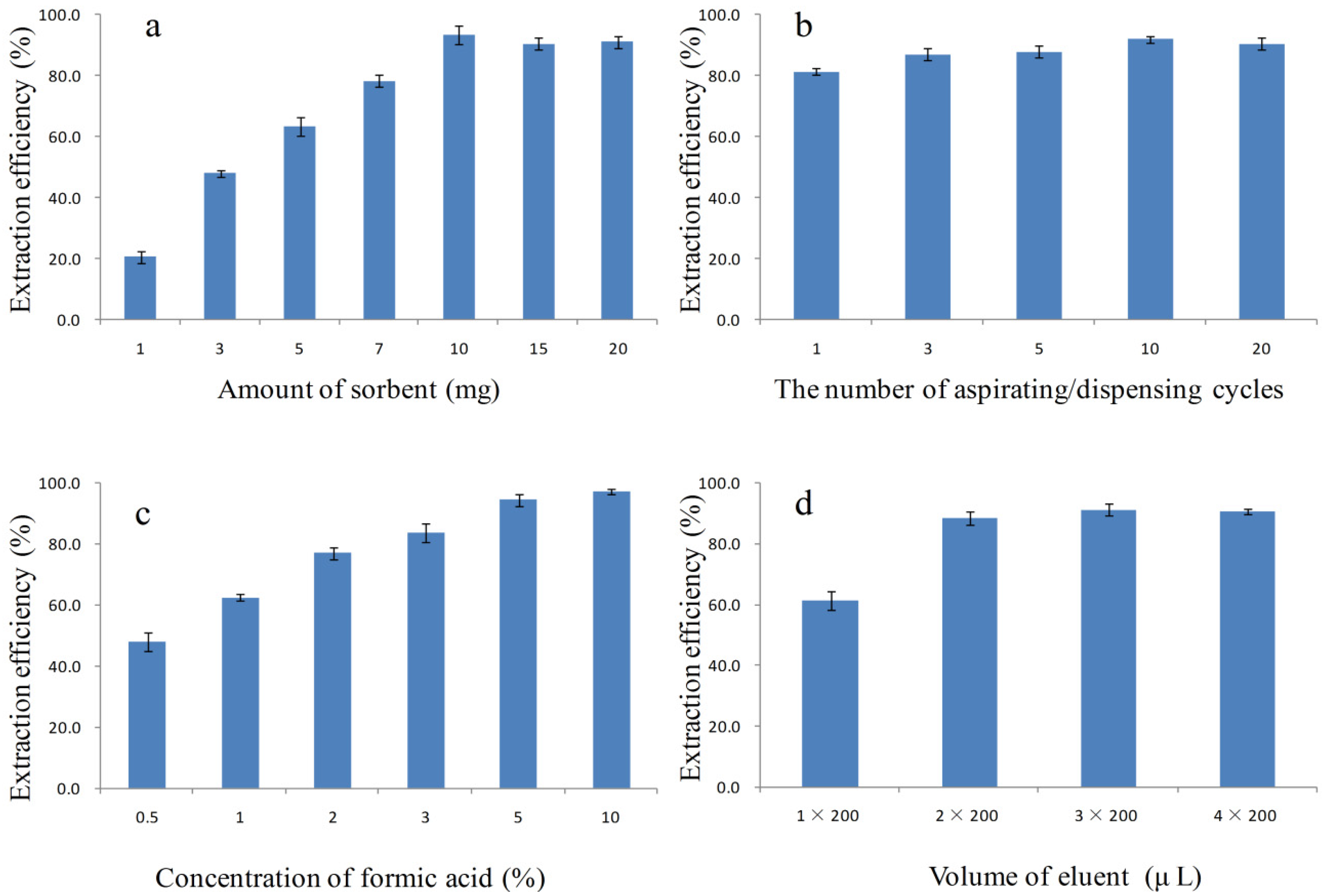
2.3. Optimization of PTSPE Procedure by the One-Factor Analysis of Variables

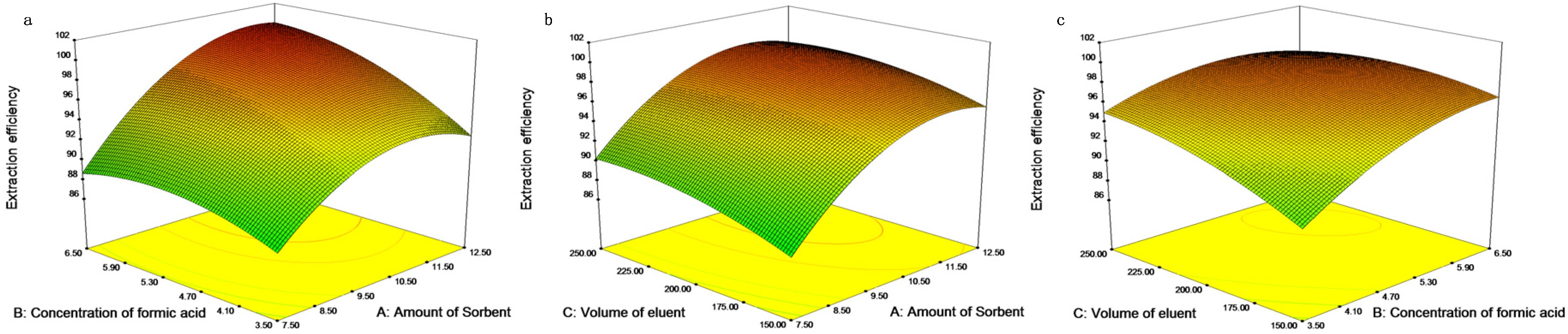
2.4. Design of Experiments
| Independent variables | Unit | Symbol | Coded Level | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |||
| Amount of sorbent | mg | A | 5 | 7.5 | 10 | 12.5 | 15 |
| Concentration of formic acid | % | B | 2 | 3.5 | 5 | 6.5 | 8 |
| Volume of eluent | μL | C | 2 × 100 | 2 × 150 | 2 × 200 | 2 × 250 | 2 × 300 |
2.5. Method Validation
| Norminal Concentration (μg/L) | Intra-Day | Inter-Day | Analyte Recovery (%, n = 6) | Matrix Effect (%, n = 3) | ||
|---|---|---|---|---|---|---|
| RSDr (%) | Biases (%) | RSDR (%) | Biases (%) | |||
| 0.37 | 7.6 | −6.5 | 10.4 | −7.4 | 88.4 ± 8.9 | 96.8 ± 2.1 |
| 0.74 | 5.8 | −4.7 | 12.7 | −5.3 | 92.8 ± 5.3 | 97.7 ± 2.6 |
| 3.7 | 2.1 | −1.9 | 3.6 | −3.1 | 97.2 ± 2.7 | 100.9 ± 1.4 |
| 37 | 2.4 | −3.1 | 2.6 | −4.2 | 102.5 ± 4.3 | 101.7 ± 1.2 |
| Norminal Concentration (μg/L) | One Week | Two Weeks | Four Weeks | |||
|---|---|---|---|---|---|---|
| Recovery (%) | Precision (%) | Recovery (%) | Precision (%) | Recovery (%) | Precision (%) | |
| 0.37 | 88.1 | 8.7 | 84.2 | 9.2 | 87.2 | 10.5 |
| 0.74 | 89.4 | 10.7 | 90.6 | 11.3 | 91.1 | 7.9 |
| 3.7 | 98.4 | 3.8 | 97.9 | 3.4 | 99.2 | 2.4 |
| 37 | 99.5 | 1.8 | 96.2 | 4.1 | 95.6 | 1.3 |
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Samples Collection and Preparation
3.3. PTSPE Procedure
3.4. Preparation of Standards and Quality Control (QC) Samples
3.5. Chromatographic Conditions
3.6. Mass Spectrometry Conditions
3.7. Method Validation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perez-Gomez, A.; Tasker, R.A. Domoic acid as a neurotoxin. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer: New York, NY, USA, 2014; pp. 399–419. [Google Scholar]
- Paredes, T.; Rietjens, I.M.C.M.; Vieites, J.M.; Cabado, A.G. Update of risk assessments of main marine biotoxins in the European Union. Toxicon 2011, 58, 336–354. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Faustman, E.M. Domoic acid as a developmental neurotoxin. Neuro Toxicol. 2010, 31, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Mos, L. Domoic acid: A fascinating marine toxin. Environ. Toxicol. Pharmacol. 2001, 9, 79–85. [Google Scholar] [CrossRef]
- Novelli, A.; Kispert, J.; Fernandez-Sanchez, M.T.; Torreblanca, A.; Zitko, V. Domoic acid-containing toxic mussels produce neurotoxicity in neuronal cultures through a synergism between excitatory amino acids. Brain Res. 1992, 577, 41–48. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Alvarez, G.; Mauriz, A.; Blanco, J. High throughput analysis of amnesic shellfish poisoning toxins in bivalve molluscs by dispersive solid-phase extraction and high-performance liquid chromatography using a monolithic column. Food Chem. 2011, 127, 1884–1891. [Google Scholar] [CrossRef]
- Mafra, L.L., Jr.; Leger, C.; Bates, S.S.; Quilliam, M.A. Analysis of trace levels of domoic acid in seawater and plankton by liquid chromatography without derivatization, using UV or mass spectrometry detection. J. Chromatogr. A 2009, 1216, 6003–6011. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, E.; Zangrando, R.; Rossi, S.; Cairns, W.R.L.; Piazza, R.; Corami, F.; Barbante, C.; Gambaro, A. Domoic acid at trace levels in lagoon waters: Assessment of a method using internal standard quantification. Anal. Bioanal. Chem. 2013, 405, 9113–9123. [Google Scholar] [CrossRef] [PubMed]
- Devez, A.; Delams, D. Selective liquid chromatographic determination of trace domoic acid in seawater and phytoplankton: Improvement using the O-phthaldialdehyde/9-fluorenylmethylchloroformate derivatization. Limnol. Oceanogr. Methods 2013, 11, 327–336. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yan, Z.M.; Gao, J.; Tong, P.; Liu, W.; Zhang, L. Metal-organic framework Uio-66 modified magnetite@silica core-shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J. Chromatogr. A 2015, 1400, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Zhang, Z.X. Quantification of domoic acid in shellfish samples by capillary electrophoresis-based enzyme immunoassay with electrochemical detection. Toxicon 2012, 59, 626–632. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 97/61/EC of 20 October. Off. J. L 1997, 295, 35–36. [Google Scholar]
- Wang, Z.H.; Maucher-Fuquay, J.; Fire, S.E.; Mikulski, C.M.; Haynes, B.; Doucette, G.J.; Ramsdell, J.S. Optimization of solid-phase extraction and liquid chromatography-tandem mass spectrometry for the determination of domoic acid in seawater, phytoplankton, and mammalian fluids and tissues. Anal. Chim. Acta 2012, 715, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Seubert, E.L.; Howard, M.D.A.; Kudela, R.M.; Stewart, T.N.; Litaker, R.W.; Evans, R.; Caron, D.A. Development, comparison, and validation using ELISAs for the determination of domoic acid in California sea Lion body fluids. J. AOAC Int. 2014, 97, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Truelove, J.; Mueller, R.; Pulido, O.; Martin, L.; Fernie, S.; Iverson, F. 30-day oral toxicity study of domoic acid in cynomolgus monkeys: Lack of overt toxicity at doses approaching the acute toxic dose. Nat. Toxins 1997, 5, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.M.; Hierlihy, S.L. Renal clearance of domoic acid in the rat. Food Chem. Toxicol. 1993, 31, 701–706. [Google Scholar] [CrossRef]
- Truelove, J.; Mueller, R.; Pulido, O.; Iverson, F. Subchronic toxicity study of domoic acid in the rat. Food Chem. Toxicol. 1996, 34, 525–529. [Google Scholar] [CrossRef]
- Tor, E.R.; Puschner, B.; Whitehead, W.E. Rapid determination of domoic acid in serum and urine by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, K.M. Laboratory diagnostics in acute poisoning: Critical overview. Clin. Chem. Lab. Med. 2010, 48, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Gonmori, K.; Minakata, K.; Suzuki, M.; Yamagishi, I.; Nozawa, H.; Hasegawa, K.; Wurita, A.; Watanabe, K.; Suzuki, O. MALDI-TOF mass spectrometric analysis of α-amanitin, β-amanitin, and phalloidin in urine. Forensic Toxicol. 2012, 30, 179–184. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Chen, L.; Li, J.H.; Liu, D.Y.; Chen, L.X. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. TrAC Trends Anal. Chem. 2014, 59, 26–41. [Google Scholar] [CrossRef]
- Wang, Z.H.; King, K.L.; Ramsdell, J.S.; Doucette, G.J. Determination of domoic acid in seawater and phytoplankton by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1163, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Beach, D.G.; Liu, H.; Quilliam, M.A. Sensitive determination of domoic acid in mussel tissue using dansyl chloride derivatization and liquid chromatography-mass spectrometry. Anal. Methods 2015, 7, 1000–1007. [Google Scholar] [CrossRef]
- Regueiro, J.; Martin-Morales, E.; Alvarez, G.; Blanco, J. Sensitive determination of domoic acid in shellfish by on-line coupling of weak anion exchange solid-phase extraction and liquid chromatography-diode array detection-tandem mass spectrometry. Food Chem. 2011, 129, 672–678. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Lau, B.P.Y.; Cleroux, C.; Lewis, D. Comparison of UV absorption and electrospray mass spectrometry for the high-performance liquid chromatographic determination of domoic acid in shellfish and biological samples. J. Chromatogr. A 1994, 659, 119–126. [Google Scholar] [CrossRef]
- Chan, I.O.M.; Tsang, V.W.H.; Chu, K.K.; Leung, S.K.; Lam, M.H.W.; Lau, T.C.; Lam, P.K.S.; Wu, R.S.S. Solid-phase extraction-fluorimetric high performance liquid chromatographic determination of domoic acid in natural seawater mediated by an amorphous titania sorbent. Anal. Chim. Acta 2007, 583, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Guo, X.C.; Zhao, H.Q.; Wu, S.X.; Yang, H.H.; Wang, X.R. Molecularly imprinted polymer for selective extraction of domoic acid from seafood coupled with high-performance liquid chromatographic determination. Talanta 2011, 84, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Dong, W.; Wang, Y.X.; Gong, L.K.; Dai, Z.Y.; Cheung, H.Y. Pipette tip solid-phase extraction and ultra-performance liquid chromatography/mass spectrometry based rapid analysis of picrosides from Picrorhiza scrophulariiflora. J. Pharm. Biomed. Anal. 2013, 80, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Svacinova, J.; Novak, O.; Plackova, L.; Lenobel, R.; Holik, J.; Strnad, M.; Dolezal, K. A new approach for cytokinin isolation from arabidopsis tissues using miniaturized purification: Pipette tip solid-phase extraction. Plant Methods 2012, 8, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.N.; Liang, S.R.; Yan, H.Y.; Ma, Z.Y.; Liu, Y.X. Ionic liquid-molecularly imprinted polymers for pipette tip solid-phase extraction of (Z)-3-(chloromethylene)-6-flourothiochroman-4-one in urine. J. Chromatogr. A 2015, 1408, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Candidoti, L.V.; de Zan, M.M.; Camara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Khodadoust, S.; Ghaedi, M. Application of response surface methodology for determination of methyl red in water samples by spectrophotometry method. Spectrochim. Acta A 2014, 133, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Talebianpoor, M.S.; Khodadoust, S.; Rozbehi, A.; Akbartabar Toori, M.; Zoladl, M.; Ghaedi, M.; Mohammadi, R.; Hosseinzadeh, A.S. Application of optimized dispersive liquid-liquid microextraction for determination of melatonin by HPLC-UV in plasma samples. J. Chromatogr. B 2014, 960, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. R. J. Stat. Soc. Ser. B 1951, 13, 1–45. [Google Scholar]
- Ma, J.P.; Xiao, R.H.; Li, J.H.; Yu, J.B.; Zhang, Y.Q.; Chen, L.X. Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 5462–5469. [Google Scholar] [CrossRef] [PubMed]
- Asgharinezhad, A.A.; Mollazadeh, N.; Ebrahimzadeh, H.; Mirbabaei, F.; Shekari, N. Magnetic nanoparticles based dispersive micro-solid-phase extraction as a novel technique for coextraction of acidic and basic drugs from biological fluids and waste water. J. Chromatogr. A 2014, 1338, 54621–54628. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, P.I.; Havlicek, L.; Vagner, M.; Malbeck, J.; Kaminek, M. Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J. Chromatogr. A 2005, 1075, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Zhao, Y.F.; Miao, H.; Wu, Y.N. A novel cation exchange polymer as a reversed-dispersive solid phase extraction sorbent for the rapid determination of rhodamine B residue in chili powder and chili oil. J. Chromatogr. A 2014, 1374, 268–272. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, D.; Hong, Z. A Rapid LC-HRMS Method for the Determination of Domoic Acid in Urine Using a Self-Assembly Pipette Tip Solid-Phase Extraction. Toxins 2016, 8, 10. https://doi.org/10.3390/toxins8010010
Zhang Y, Chen D, Hong Z. A Rapid LC-HRMS Method for the Determination of Domoic Acid in Urine Using a Self-Assembly Pipette Tip Solid-Phase Extraction. Toxins. 2016; 8(1):10. https://doi.org/10.3390/toxins8010010
Chicago/Turabian StyleZhang, Yiping, Dawei Chen, and Zhuan Hong. 2016. "A Rapid LC-HRMS Method for the Determination of Domoic Acid in Urine Using a Self-Assembly Pipette Tip Solid-Phase Extraction" Toxins 8, no. 1: 10. https://doi.org/10.3390/toxins8010010






