Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin
Abstract
:1. Introduction
2. Role of C-Terminal Domain in the Binding of Alpha-Toxin to Membrane
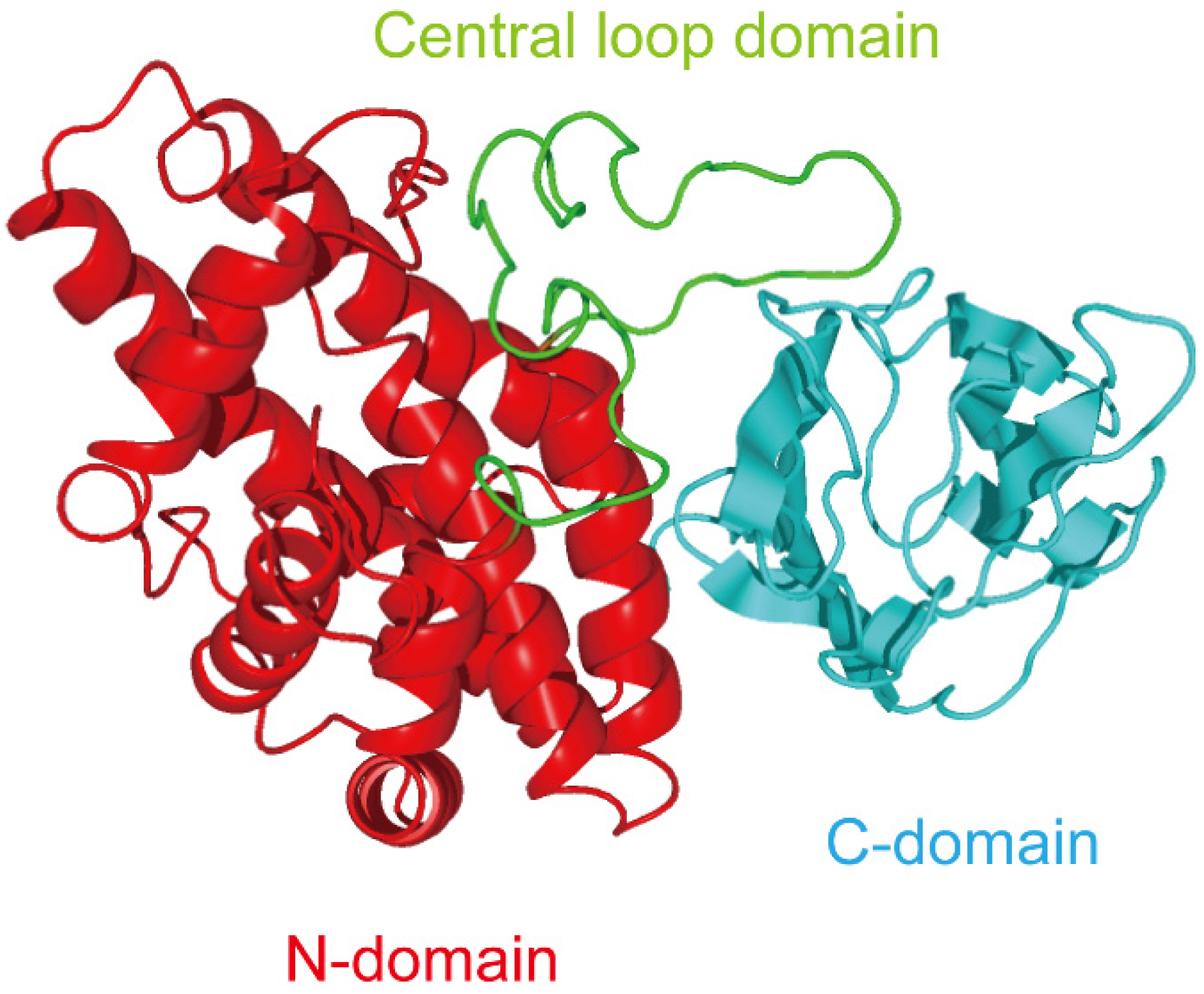
3. Exploitation of C-Terminal Domain of Alpha-Toxin
4. Relationship between Alpha-Toxin and Gangliosides
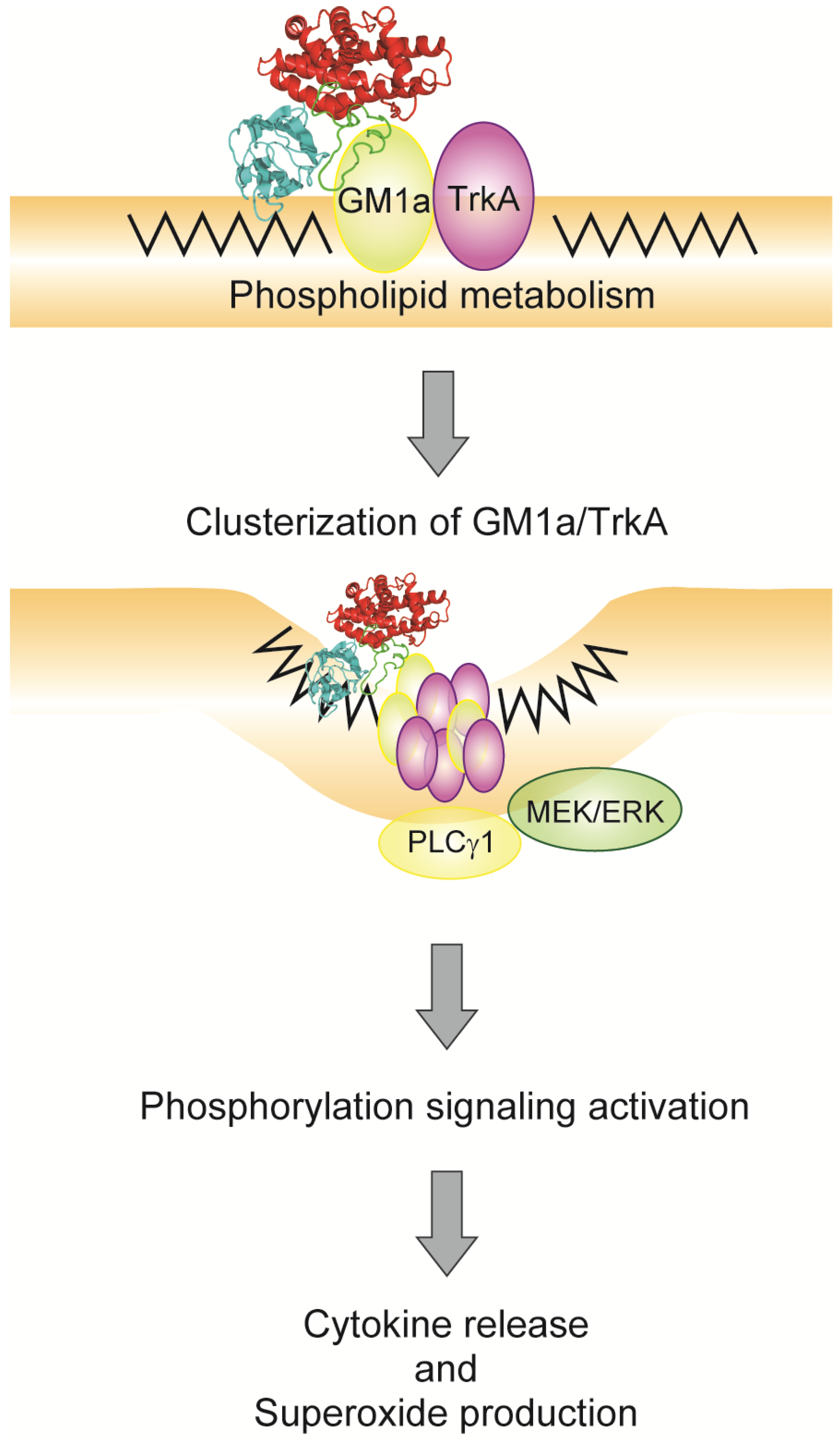
5. Endocytosis of Alpha-Toxin in Ganglioside Deficient Cells
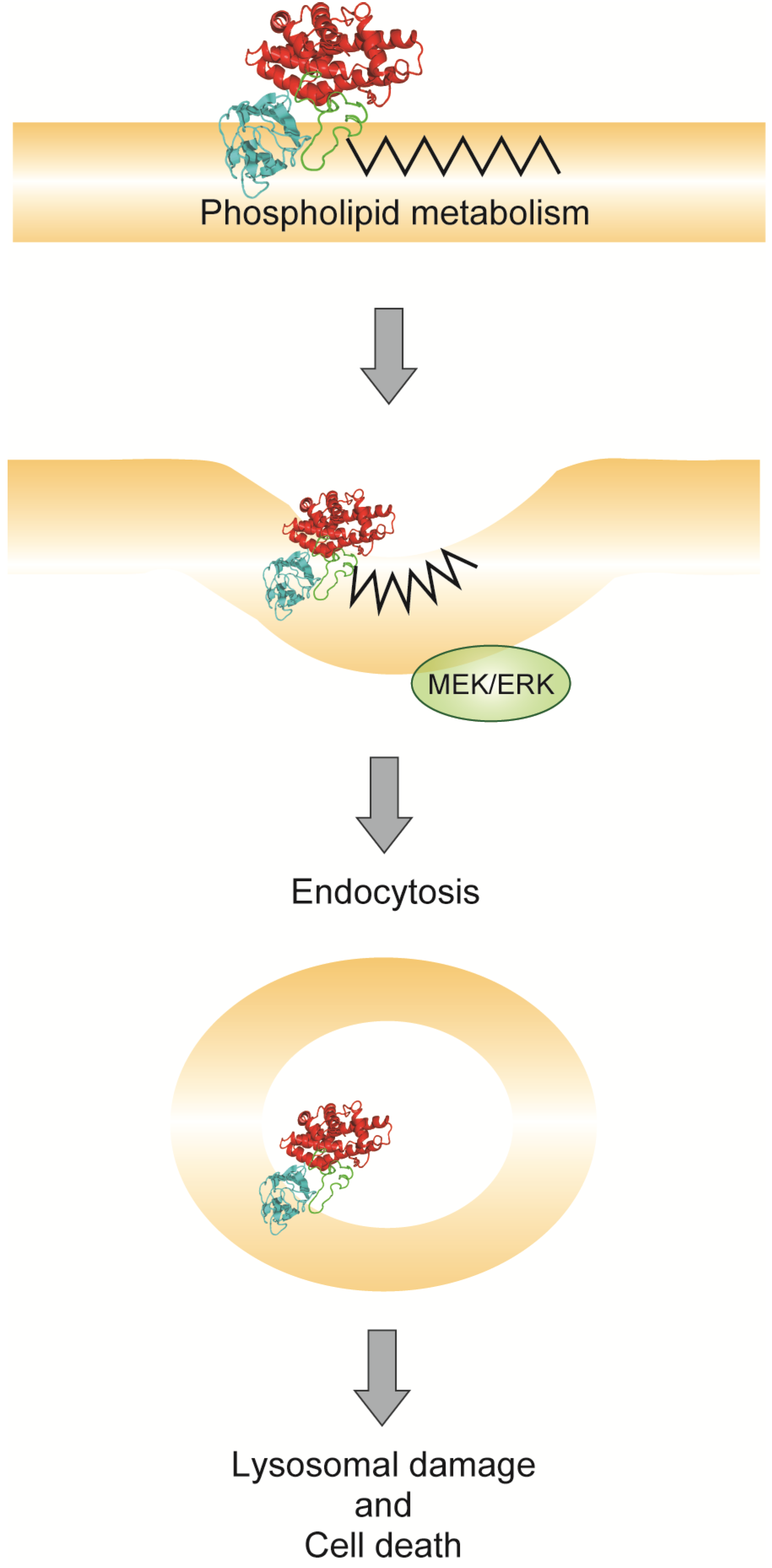
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maclennan, J.D. The histotoxic clostridial infections of man. Bacteriol. Rev. 1962, 26, 177–276. [Google Scholar] [PubMed]
- Bryant, A.E.; Chen, R.Y.; Nagata, Y.; Wang, Y.; Lee, C.H.; Finegold, S.; Guth, P.H.; Stevens, D.L. Clostridial gas gangrene. I. Cellular and molecular mechanisms of microvascular dysfunction induced by exotoxins of Clostridium perfringens. J. Infect. Dis. 2000, 182, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Chen, R.Y.; Nagata, Y.; Wang, Y.; Lee, C.H.; Finegold, S.; Guth, P.H.; Stevens, D.L. Clostridial gas gangrene. II. Phospholipase C—Induced activation of platelet gpiibiiia mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J. Infect. Dis. 2000, 182, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Nagahama, M.; Oda, M. Clostridium perfringens alpha-toxin: Characterization and mode of action. J. Biochem. 2004, 136, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Ikari, S.; Matsuno, T.; Morimune, Y.; Nagahama, M.; Sakurai, J. Signal transduction mechanism involved in Clostridium perfringens alpha-toxin-induced superoxide anion generation in rabbit neutrophils. Infect. Immun. 2006, 74, 2876–2886. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kihara, A.; Yoshioka, H.; Saito, Y.; Watanabe, N.; Uoo, K.; Higashihara, M.; Nagahama, M.; Koide, N.; Yokochi, T.; et al. Effect of erythromycin on biological activities induced by Clostridium perfringens alpha-toxin. J. Pharmacol. Exp. Ther. 2008, 327, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Oda, M.; Matsuda, H.; Ikari, S.; Sakurai, J. Clostridium perfringens alpha-toxin activates the sphingomyelin metabolism system in sheep erythrocytes. J. Biol. Chem. 2004, 279, 12181–12189. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. The Comprehensive Sourcebook of Bacterial Protein Toxins, 2nd ed.; Academic Press: London, UK, 1999; pp. 623–636. [Google Scholar]
- Titball, R.W. Bacterial phospholipases C. Microbiol. Rev. 1993, 57, 347–366. [Google Scholar] [PubMed]
- Naylor, C.E.; Eaton, J.T.; Howells, A.; Justin, N.; Moss, D.S.; Titball, R.W.; Basak, A.K. Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 1998, 5, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Chahinian, H.; Sias, B.; Carriere, F. The C-terminal domain of pancreatic lipase: Functional and structural analogies with C2 domains. Curr. Protein Pept. Sci. 2000, 1, 91–103. [Google Scholar] [PubMed]
- Titball, R.W.; Naylor, C.E.; Miller, J.; Moss, D.S.; Basak, A.K. Opening of the active site of Clostridium perfringens alpha-toxin may be triggered by membrane binding. Int. J. Med. Microbiol. 2000, 290, 357–361. [Google Scholar] [CrossRef]
- Hough, E.; Hansen, L.K.; Birknes, B.; Jynge, K.; Hansen, S.; Hordvik, A.; Little, C.; Dodson, E.; Derewenda, Z. High-resolution (1.5 Å) crystal structure of phospholipase C from bacillus cereus. Nature 1989, 338, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Sandford, R. The PLAT domain: A new piece in the PKD1 puzzle. Curr. Biol. 1999, 9, R588–R590. [Google Scholar] [CrossRef]
- Naylor, C.E.; Jepson, M.; Crane, D.T.; Titball, R.W.; Miller, J.; Basak, A.K.; Bolgiano, B. Characterisation of the calcium-binding C-terminal domain of Clostridium perfringens alpha-toxin. J. Mol. Biol. 1999, 294, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Jepson, M.; Bullifent, H.L.; Crane, D.; Flores-Diaz, M.; Alape-Giron, A.; Jayasekeera, P.; Lingard, B.; Moss, D.; Titball, R.W. Tyrosine 331 and phenylalanine 334 in Clostridium perfringens alpha-toxin are essential for cytotoxic activity. FEBS Lett. 2001, 495, 172–177. [Google Scholar] [CrossRef]
- Alape-Giron, A.; Flores-Diaz, M.; Guillouard, I.; Naylor, C.E.; Titball, R.W.; Rucavado, A.; Lomonte, B.; Basak, A.K.; Gutierrez, J.M.; Cole, S.T.; et al. Identification of residues critical for toxicity in Clostridium perfringens phospholipase c, the key toxin in gas gangrene. Eur. J. Biochem. 2000, 267, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Mukai, M.; Morimitsu, S.; Ochi, S.; Sakurai, J. Role of the C-domain in the biological activities of Clostridium perfringens alpha-toxin. Microbiol. Immunol. 2002, 46, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Otsuka, A.; Oda, M.; Singh, R.K.; Ziora, Z.M.; Imagawa, H.; Nishizawa, M.; Sakurai, J. Effect of unsaturated bonds in the sn-2 acyl chain of phosphatidylcholine on the membrane-damaging action of Clostridium perfringens alpha-toxin toward liposomes. Biochim. Biophys. Acta 2007, 1768, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.D.; Titball, R.W. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene. Vaccine 1993, 11, 1253–1258. [Google Scholar] [CrossRef]
- Titball, R.W.; Fearn, A.M.; Williamson, E.D. Biochemical and immunological properties of the C-terminal domain of the alpha-toxin of Clostridium perfringens. FEMS Microbiol. Lett. 1993, 110, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Titball, R.W.; Jepson, M.; Bayer, C.R.; Hayes-Schroer, S.M.; Bryant, A.E. Immunization with the C-domain of alpha -toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 2004, 190, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Shreya, D.; Uppalapati, S.R.; Kingston, J.J.; Sripathy, M.H.; Batra, H.V. Immunization with recombinant bivalent chimera r-Cpae confers protection against alpha toxin and enterotoxin of Clostridium perfringens type A in murine model. Mol. Immunol. 2015, 65, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Oda, M.; Kobayashi, K.; Ochi, S.; Takagishi, T.; Shibutani, M.; Sakurai, J. A recombinant carboxy-terminal domain of alpha-toxin protects mice against Clostridium perfringens. Microbiol. Immunol. 2013, 57, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M. Vaccines against Clostridium perfringens alpha-toxin. Curr. Pharm. Biotechnol. 2013, 14, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.T.; Naylor, C.E.; Howells, A.M.; Moss, D.S.; Titball, R.W.; Basak, A.K. Crystal structure of the C. perfringens alpha-toxin with the active site closed by a flexible loop region. J. Mol. Biol. 2002, 319, 275–281. [Google Scholar] [CrossRef]
- Clark, G.C.; Briggs, D.C.; Karasawa, T.; Wang, X.; Cole, A.R.; Maegawa, T.; Jayasekera, P.N.; Naylor, C.E.; Miller, J.; Moss, D.S.; et al. Clostridium absonum alpha-toxin: New insights into clostridial phospholipase C substrate binding and specificity. J. Mol. Biol. 2003, 333, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Kozai, Y.; Ihara, H.; Kohda, T.; Mukamoto, M.; Tsuji, T.; Kozaki, S. Identification of the receptor-binding sites in the carboxyl-terminal half of the heavy chain of botulinum neurotoxin types C and D. Microb. Pathog. 2008, 44, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kabura, M.; Takagishi, T.; Suzue, A.; Tominaga, K.; Urano, S.; Nagahama, M.; Kobayashi, K.; Furukawa, K.; Sakurai, J. Clostridium perfringens alpha-toxin recognizes the GM1a-Trka complex. J. Biol. Chem. 2012, 287, 33070–33079. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, T.; Tokuda, A.; Inokuchi, J.; Kuriyama, M. Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 1998, 273, 26001–26007. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Horibata, Y.; Nagatsuka, Y.; Hirabayashi, Y.; Hashikawa, T. Fucoganglioside alpha-fucosyl(alpha-galactosyl)-GM1: A novel member of lipid membrane microdomain components involved in PC12 cell neuritogenesis. Biochem. J. 2007, 407, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.M.; Ren, Q.; Mo, L.; Neff, N.H.; Hadjiconstantinou, M. GM1 ganglioside induces phosphorylation and activation of Trk and Erk in brain. J. Neurochem. 2002, 81, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Iwabuchi, K.; Kurihara, H.; Ishii, K.; Kobayashi, T.; Sasaki, T.; Hattori, N.; Mizuno, Y.; Hozumi, K.; Yamada, Y.; et al. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J. Cell Sci. 2009, 122, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, T.; Oda, M.; Kabura, M.; Kurosawa, M.; Tominaga, K.; Urano, S.; Ueda, Y.; Kobayashi, K.; Kobayashi, T.; Sakurai, J.; et al. Clostridium perfringens alpha-toxin induces GM1a clustering and TrkA phosphorylation in the host cell membrane. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Makino, A.; Murase-Tamada, K.; Sakai, S.; Inaba, T.; Hullin-Matsuda, F.; Kobayashi, T. Sphingomyelin regulates the transbilayer movement of diacylglycerol in the plasma membrane of madin-darby canine kidney cells. FASEB J. 2013, 27, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, P.; Dupuy, J.; Imamura, A.; Kiso, M.; Stevens, R.C. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog. 2008, 4, e1000129. [Google Scholar] [CrossRef] [PubMed]
- Strotmeier, J.; Lee, K.; Volker, A.K.; Mahrhold, S.; Zong, Y.; Zeiser, J.; Zhou, J.; Pich, A.; Bigalke, H.; Binz, T.; et al. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 2010, 431, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Karalewitz, A.P.; Kroken, A.R.; Fu, Z.; Baldwin, M.R.; Kim, J.J.; Barbieri, J.T. Identification of a unique ganglioside binding loop within botulinum neurotoxins C and D-SA. Biochemistry 2010, 49, 8117–8126. [Google Scholar] [CrossRef] [PubMed]
- Jepson, M.; Howells, A.; Bullifent, H.L.; Bolgiano, B.; Crane, D.; Miller, J.; Holley, J.; Jayasekera, P.; Titball, R.W. Differences in the carboxy-terminal (putative phospholipid binding) domains of Clostridium perfringens and Clostridium bifermentans phospholipases C influence the hemolytic and lethal properties of these enzymes. Infect. Immun. 1999, 67, 3297–3301. [Google Scholar] [PubMed]
- Monturiol-Gross, L.; Flores-Diaz, M.; Campos-Rodriguez, D.; Mora, R.; Rodriguez-Vega, M.; Marks, D.L.; Alape-Giron, A. Internalization of Clostridium perfringens alpha-toxin leads to ERK activation and is involved on its cytotoxic effect. Cell Microbiol. 2014, 16, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Matsuno, T.; Shiihara, R.; Ochi, S.; Yamauchi, R.; Saito, Y.; Imagawa, H.; Nagahama, M.; Nishizawa, M.; Sakurai, J. The relationship between the metabolism of sphingomyelin species and the hemolysis of sheep erythrocytes induced by Clostridium perfringens alpha-toxin. J. Lipid Res. 2008, 49, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kinoshita, T. GPI-anchor remodeling: Potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim. Biophys. Acta 2012, 1821, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Pust, S.; Skotland, T.; van Deurs, B. Clathrin-independent endocytosis: Mechanisms and function. Curr. Opin. Cell Biol. 2011, 23, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.T.; Kirkham, M.; Riches, J.; Cortese, K.; Walser, P.J.; Simpson, F.; Hill, M.M.; Jones, A.; Lundmark, R.; Lindsay, M.R.; et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010, 190, 675–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.C.; Xiong, W.; Zheng, J.; Zhou, Y.; Zheng, H.; Zhang, C.; Zheng, L.H.; Zhu, X.L.; Xiong, Z.Q.; Wang, L.Y.; et al. The timing of endocytosis after activation of a G-protein-coupled receptor in a sensory neuron. Biophys. J. 2006, 90, 3590–3598. [Google Scholar] [CrossRef] [PubMed]
- Urbina, P.; Collado, M.I.; Alonso, A.; Goni, F.M.; Flores-Diaz, M.; Alape-Giron, A.; Ruysschaert, J.M.; Lensink, M.F. Unexpected wide substrate specificity of C. perfringens alpha-toxin phospholipase C. Biochim. Biophys. Acta 2011, 1808, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Almena, M.; Merida, I. Shaping up the membrane: Diacylglycerol coordinates spatial orientation of signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Stancevic, B.; Kolesnick, R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010, 584, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Michiue, K.; Mukai, M.; Ochi, S.; Sakurai, J. Mechanism of membrane damage by Clostridium perfringens alpha-toxin. Microbiol. Immunol. 1998, 42, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Hashimoto, K.; Nagahama, M.; Sakurai, J. Phospholipid metabolism induced by Clostridium perfringens alpha-toxin elicits a hot-cold type of hemolysis in rabbit erythrocytes. Infect. Immun. 1996, 64, 3930–3933. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oda, M.; Terao, Y.; Sakurai, J.; Nagahama, M. Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin. Toxins 2015, 7, 5268-5275. https://doi.org/10.3390/toxins7124880
Oda M, Terao Y, Sakurai J, Nagahama M. Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin. Toxins. 2015; 7(12):5268-5275. https://doi.org/10.3390/toxins7124880
Chicago/Turabian StyleOda, Masataka, Yutaka Terao, Jun Sakurai, and Masahiro Nagahama. 2015. "Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin" Toxins 7, no. 12: 5268-5275. https://doi.org/10.3390/toxins7124880





