Molecular Surface of JZTX-V (β-Theraphotoxin-Cj2a) Interacting with Voltage-Gated Sodium Channel Subtype NaV1.4
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selectivity of JZTX-V for Voltage-Gated Sodium Channel Subtypes

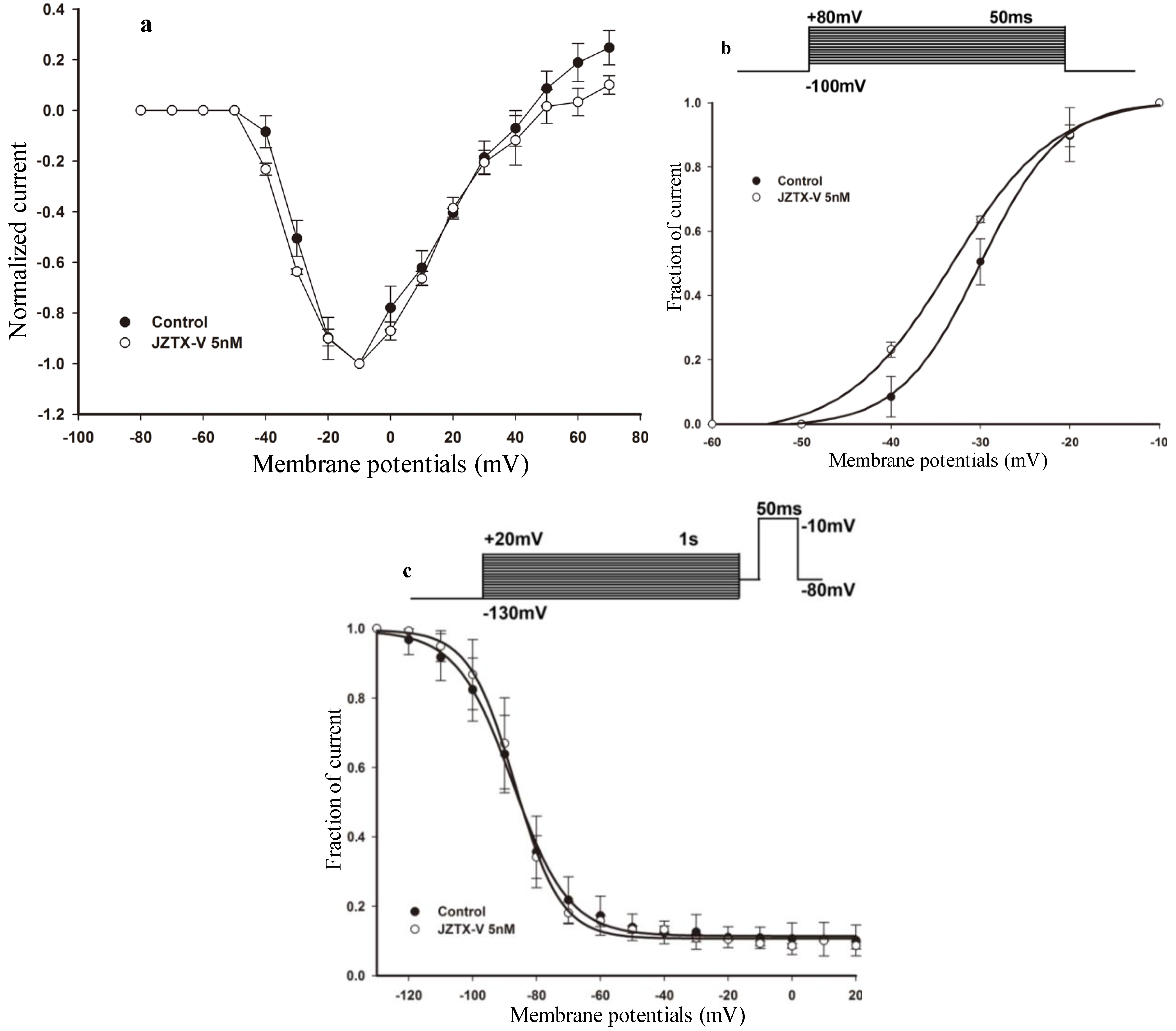
2.2. Effects of Subsaturating Concentrations of JZTX-V on Activation and Inactivation Properties of the Wild-Type VGSC Subtype NaV1.4
2.3. Synthesis and Structural Integrity of JZTX-V
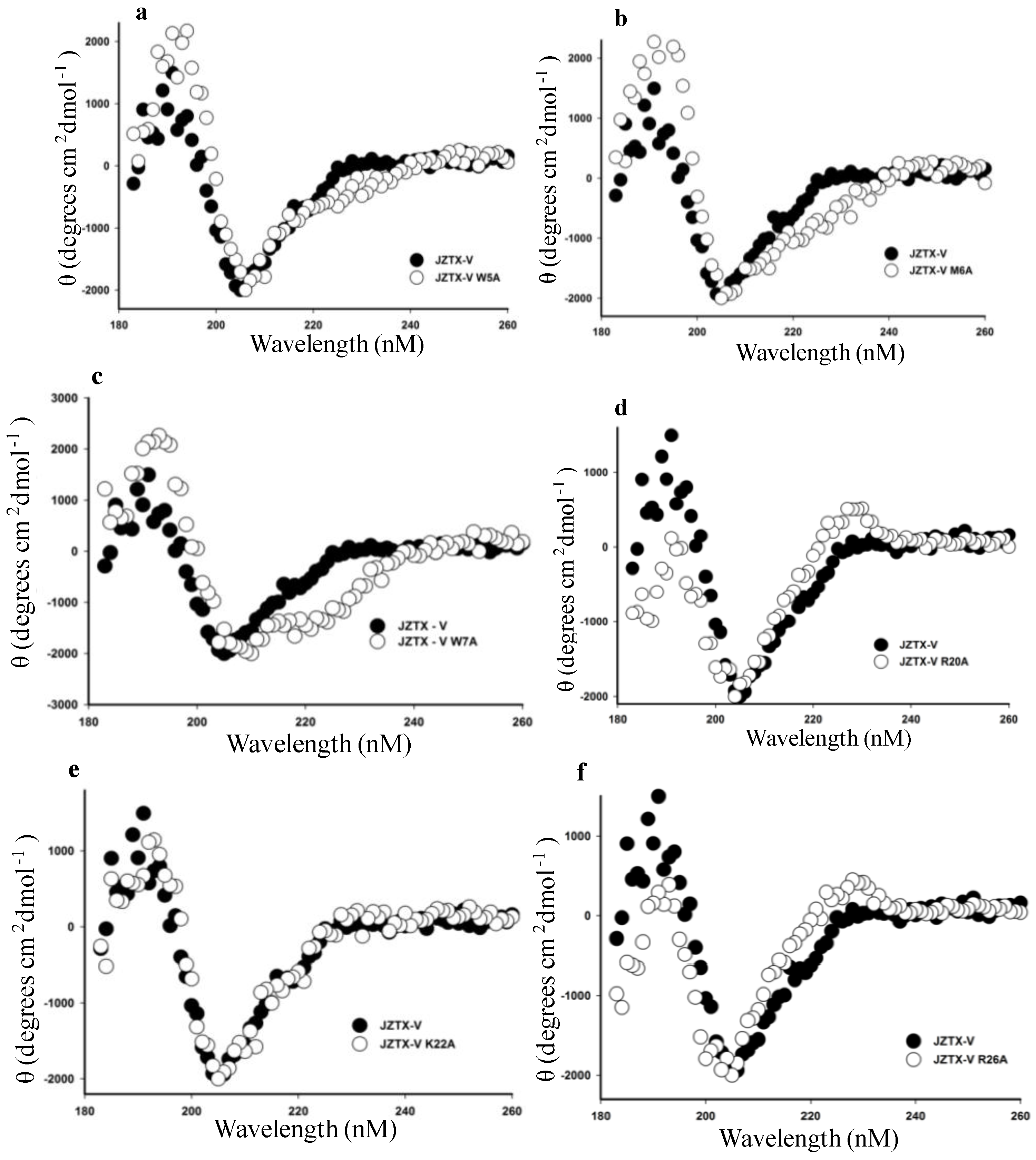
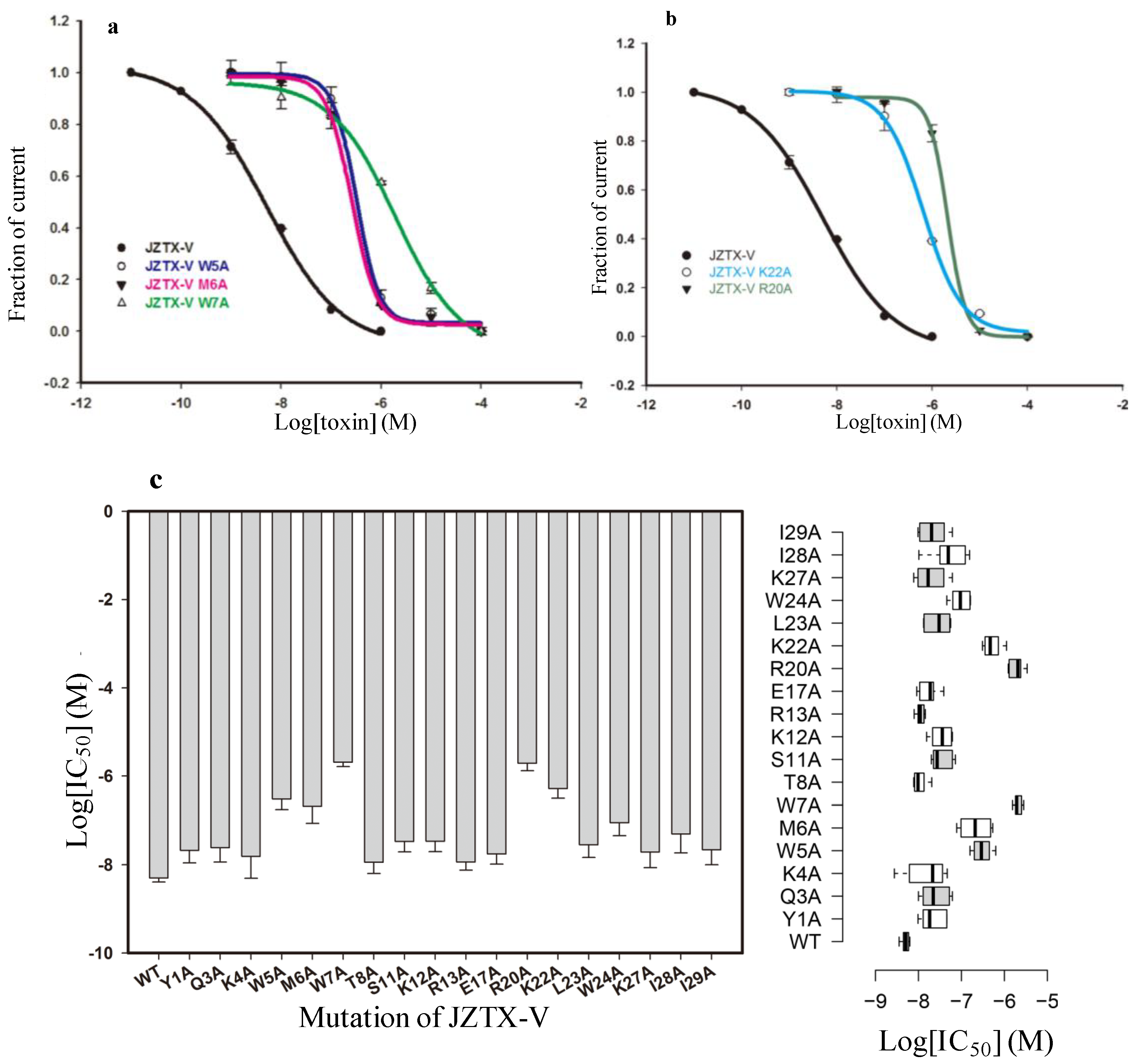
2.4. Functional Characterization of Mutant Forms of JZTX-V
2.5. Discussion
2.5.1. JZTX-V Is a Selective Antagonist of Voltage-Gated Sodium Channel Subtype NaV1.4
2.5.2. Effect of JZTX-V on Voltage-Gated Sodium Channel Subtype NaV1.4
2.5.3. The Bioactive Surface of JZTX-V on Voltage-Gated Sodium Channel Subtype NaV1.4

2.5.4. Comparison with Other Spider Toxins Which Have Effects on Voltage-Gated Sodium Channel Subtype NaV1.4
3. Experimental Section
3.1. Peptide Synthesis and Oxidation
3.2. Transient Transfection
3.3. Whole-Cell Patch-Clamp Recording
3.4. Toxin Solutions and Bath Application
3.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hodgkin, A.; Huxley, A. The components of membrane conductance in the giant axon of loligo. J. Physiol. 1952, 116, 473–496. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. The dual effect of membrane potential on sodium conductance in the giant axon of loligo. J. Physiol. 1952, 116, 497–506. [Google Scholar]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef]
- Isom, L.L. Sodium channel β subunits: Anything but auxiliary. Neuroscientist 2001, 7, 42–54. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage—Gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012, 590, 2577–2589. [Google Scholar] [CrossRef]
- George, A.L.; Knittle, T.J.; Tamkun, M.M. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: Evidence for a distinct gene family. Proc. Natl. Acad. Sci. USA 1992, 89, 4893–4897. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International union of pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Westenbroek, R.E.; Merrick, D.K.; Catterall, W.A. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron 1989, 3, 695–704. [Google Scholar] [CrossRef]
- Boiko, T.; Rasband, M.N.; Levinson, S.R.; Caldwell, J.H.; Mandel, G.; Trimmer, J.S.; Matthews, G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 2001, 30, 91–104. [Google Scholar] [CrossRef]
- Kaplan, M.R.; Cho, M.-H.; Ullian, E.M.; Isom, L.L.; Levinson, S.R.; Barres, B.A. Differential control of clustering of the sodium channels NaV1.2 and NaV1.6 at developing CNS nodes of Ranvier. Neuron 2001, 30, 105–119. [Google Scholar] [CrossRef]
- Beckh, S.; Noda, M.; Lübbert, H.; Numa, S. Differential regulation of three sodium channel messenger rnas in the rat central nervous system during development. EMBO J. 1989, 8, 3611–3616. [Google Scholar]
- Whitaker, W.; Faull, R.; Waldvogel, H.; Plumpton, C.; Burbidge, S.; Emson, P.; Clare, J. Localization of the type VI voltage-gated sodium channel protein in human CNS. Neuroreport 1999, 10, 3703–3709. [Google Scholar] [CrossRef]
- Tzoumaka, E.; Tischler, A.C.; Sangameswaran, L.; Eglen, R.M.; Hunter, J.C.; Novakovic, S.D. Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system. J. Neurosci. Res. 2000, 60, 37–44. [Google Scholar] [CrossRef]
- Caldwell, J.H.; Schaller, K.L.; Lasher, R.S.; Peles, E.; Levinson, S.R. Sodium channel NaV1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. USA 2000, 97, 5616–5620. [Google Scholar] [CrossRef]
- Sangameswaran, L.; Fish, L.M.; Koch, B.D.; Rabert, D.K.; Delgado, S.G.; Ilnicka, M.; Jakeman, L.B.; Novakovic, S.; Wong, K.; Sze, P. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J. Biol. Chem. 1997, 272, 14805–14809. [Google Scholar] [CrossRef]
- Klugbauer, N.; Lacinova, L.; Flockerzi, V.; Hofmann, F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995, 14, 1084–1090. [Google Scholar]
- Rogart, R.; Cribbs, L.; Muglia, L.; Kephart, D.; Kaiser, M. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc. Natl. Acad. Sci. USA 1989, 86, 8170–8174. [Google Scholar] [CrossRef]
- Trimmer, J.S.; Cooperman, S.S.; Agnew, W.S.; Mandel, G. Regulation of muscle sodium channel transcripts during development and in response to denervation. Dev. Biol. 1990, 142, 360–367. [Google Scholar] [CrossRef]
- Cannon, S.C. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu. Rev. Neurosci. 2006, 29, 387–415. [Google Scholar] [CrossRef]
- Coddington, J.A.; Levi, H.W. Systematics and evolution of spiders (Araneae). Annu. Rev. Ecol. Syst. 1991, 22, 565–592. [Google Scholar]
- King, G.F. The wonderful world of spiders: Preface to the special toxicon issue on spider venoms. Toxicon 2004, 43, 471–475. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef]
- Billen, B.; Bosmans, F.; Tytgat, J. Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Des. 2008, 14, 2492–2502. [Google Scholar] [CrossRef]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef]
- Herzig, V.; Wood, D.L.; Newell, F.; Chaumeil, P.-A.; Kaas, Q.; Binford, G.J.; Nicholson, G.M.; Gorse, D.; King, G.F. Arachnoserver 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011, 39, D653–D657. [Google Scholar] [CrossRef]
- Klint, J.K.; Senff, S.; Rupasinghe, D.B.; Er, S.Y.; Herzig, V.; Nicholson, G.M.; King, G.F. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 2012, 60, 478–491. [Google Scholar] [CrossRef]
- Zeng, X.; Deng, M.; Lin, Y.; Yuan, C.; Pi, J.; Liang, S. Isolation and characterization of Jingzhaotoxin-V, a novel neurotoxin from the venom of the spider Chilobrachys jingzhao. Toxicon 2007, 49, 388–399. [Google Scholar] [CrossRef]
- Bosmans, F.; Rash, L.; Zhu, S.; Diochot, S.; Lazdunski, M.; Escoubas, P.; Tytgat, J. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol. Pharmacol. 2006, 69, 419–429. [Google Scholar]
- Middleton, R.E.; Warren, V.A.; Kraus, R.L.; Hwang, J.C.; Liu, C.J.; Dai, G.; Brochu, R.M.; Kohler, M.G.; Gao, Y.-D.; Garsky, V.M. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry 2002, 41, 14734–14747. [Google Scholar] [CrossRef]
- Smith, J.J.; Cummins, T.R.; Alphy, S.; Blumenthal, K.M. Molecular interactions of the gating modifier toxin protx-ii with NaV1.5: Implied existence of a novel toxin binding site coupled to activation. J. Biol. Chem. 2007, 282, 12687–12697. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a voltage-dependent k+ channel. Nature 2003, 423, 33–41. [Google Scholar] [CrossRef]
- Lee, S.-Y.; MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 2004, 430, 232–235. [Google Scholar] [CrossRef]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Debaveye, S.; Tytgat, J.; Grishin, E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider agelena orientalis. J. Biol. Chem. 2010, 285, 18545–18554. [Google Scholar]
- Cummins, T.R.; Aglieco, F.; Renganathan, M.; Herzog, R.I.; Dib-Hajj, S.D.; Waxman, S.G. NaV1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 2001, 21, 5952–5961. [Google Scholar]
- Rush, A.M.; Dib-Hajj, S.D.; Liu, S.; Cummins, T.R.; Black, J.A.; Waxman, S.G. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 8245–8250. [Google Scholar]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The NaV1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef]
- Gilchrist, J.; Das, S.; van Petegem, F.; Bosmans, F. Crystallographic insights into sodium-channel modulation by the β4 subunit. Proc. Natl. Acad. Sci. USA 2013, 110, E5016–E5024. [Google Scholar] [CrossRef]
- Maggio, F.; King, G.F. Scanning mutagenesis of a janus-faced atracotoxin reveals a bipartite surface patch that is essential for neurotoxic function. J. Biol. Chem. 2002, 277, 22806–22813. [Google Scholar] [CrossRef]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Tytgat, J.; Grishin, E. Two novel sodium channel inhibitors from Heriaeus melloteei spider venom differentially interacting with mammalian channel’s isoforms. Toxicon 2008, 52, 309–317. [Google Scholar] [CrossRef]
- Zeng, X.-Z.; Deng, M.-C.; Sun, Z.-H.; Wang, X.-C.; Liang, S.-P. Synthesis, refolding and characterization of JZTX-V and its effect on potassium channels. Chin. J. Biochem. Mol. Biol. 2008, 24, 463–468. [Google Scholar]
- Krzywinski, M.; Altman, N. Points of significance: Visualizing samples with box plots. Nat. Methods 2014, 11, 119–120. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, J.; Zhang, Y.; Gong, M.; Lu, S.; Ma, Y.; Zeng, X.; Liang, S. Molecular Surface of JZTX-V (β-Theraphotoxin-Cj2a) Interacting with Voltage-Gated Sodium Channel Subtype NaV1.4. Toxins 2014, 6, 2177-2193. https://doi.org/10.3390/toxins6072177
Luo J, Zhang Y, Gong M, Lu S, Ma Y, Zeng X, Liang S. Molecular Surface of JZTX-V (β-Theraphotoxin-Cj2a) Interacting with Voltage-Gated Sodium Channel Subtype NaV1.4. Toxins. 2014; 6(7):2177-2193. https://doi.org/10.3390/toxins6072177
Chicago/Turabian StyleLuo, Ji, Yiya Zhang, Mengting Gong, Shanshan Lu, Yifeng Ma, Xiongzhi Zeng, and Songping Liang. 2014. "Molecular Surface of JZTX-V (β-Theraphotoxin-Cj2a) Interacting with Voltage-Gated Sodium Channel Subtype NaV1.4" Toxins 6, no. 7: 2177-2193. https://doi.org/10.3390/toxins6072177
APA StyleLuo, J., Zhang, Y., Gong, M., Lu, S., Ma, Y., Zeng, X., & Liang, S. (2014). Molecular Surface of JZTX-V (β-Theraphotoxin-Cj2a) Interacting with Voltage-Gated Sodium Channel Subtype NaV1.4. Toxins, 6(7), 2177-2193. https://doi.org/10.3390/toxins6072177



