Presence of Potential Toxin-Producing Cyanobacteria in an Oligo-Mesotrophic Lake in Baltic Lake District, Germany: An Ecological, Genetic and Toxicological Survey
Abstract
:1. Introduction
2. Results
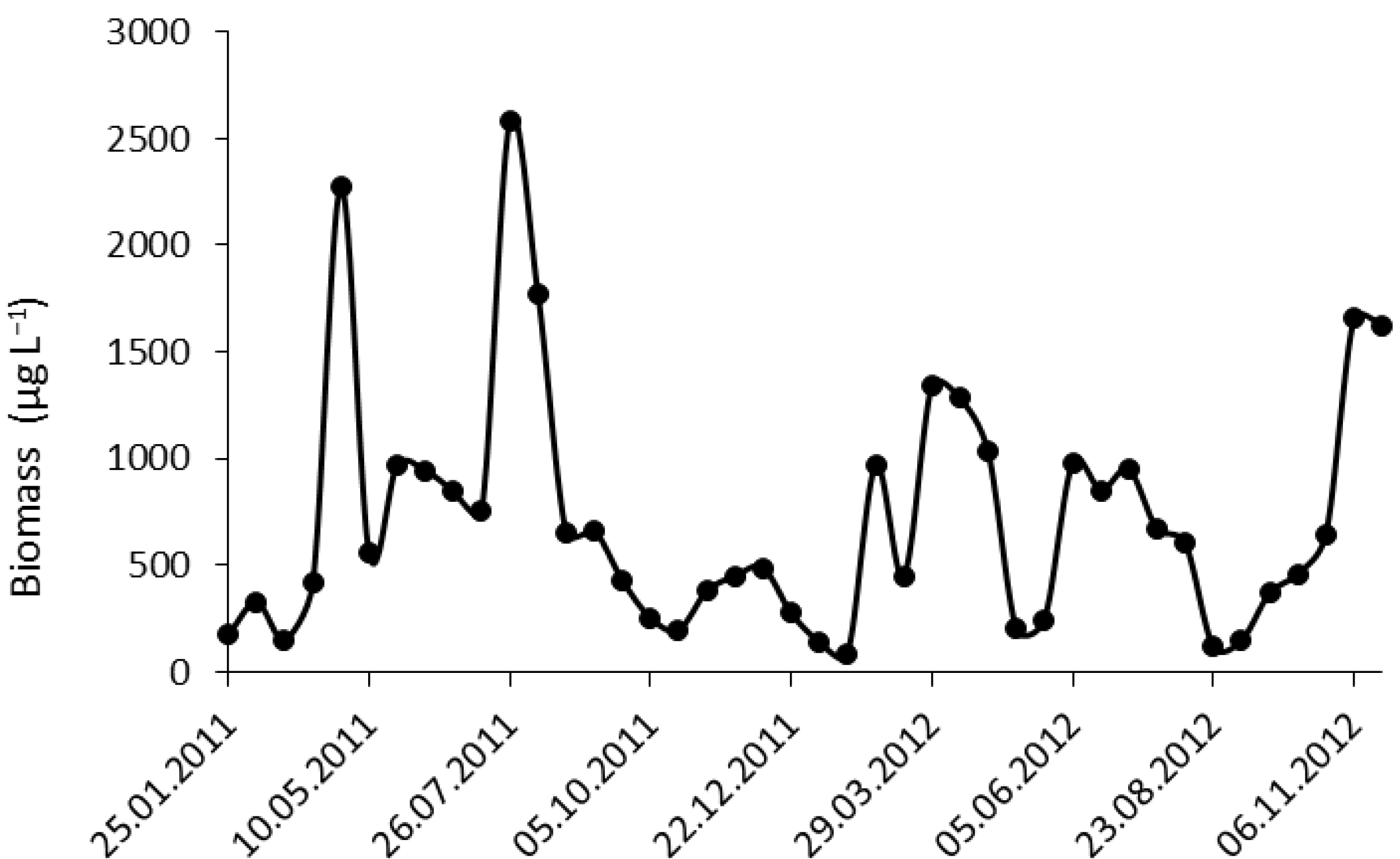
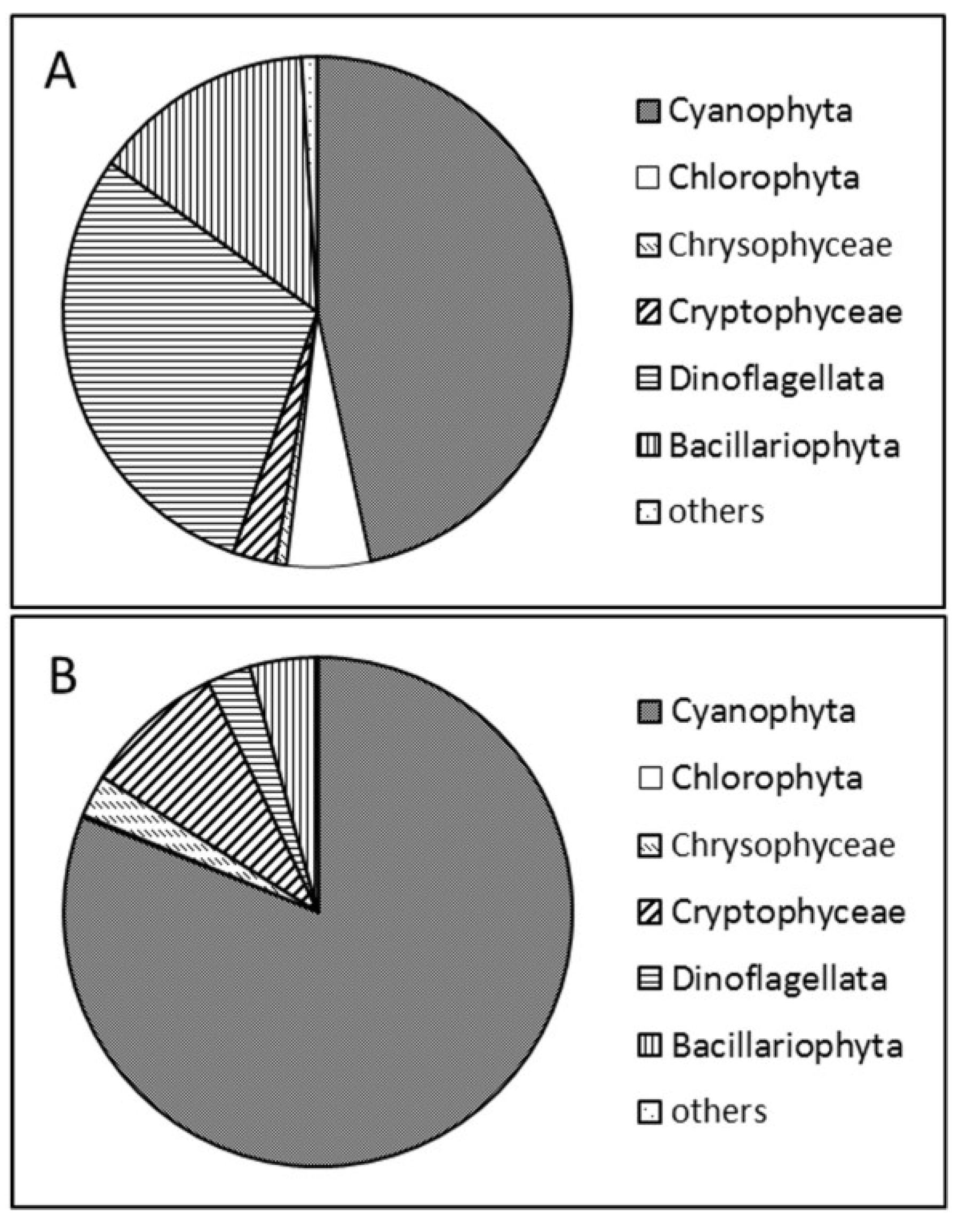
2.1. Phytoplankton Study
| Sampling time | Water Layers | TP (mg·L−1) | SRP (mg·L−1) | TN (mg·L−1) | DIN (mg·L−1) | pH | Conductivity (µS·cm−1) | O2 (mg·L−1) | O2 (%) | Secchi depth (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| July 2011 | Epilimnion | 0.011 | 0.002 | 0.376 | 0.039 | 8.70 | 285 | 10.87 | 122.4 | 5.7 |
| Hypolimnion | 0.020 | 0.009 | 0.334 | 0.064 | 8.17 | 292 | 11.99 | 99.5 | ||
| Nov 2012 | Epilimnion | 0.010 | 0.003 | 0.393 | 0.024 | 7.92 | 282 | 10.52 | 91.3 | 5.5 |
| Hypolimnion | 0.113 | 0.104 | 0.425 | 0.155 | 7.02 | 298 | 4.27 | 34.0 |

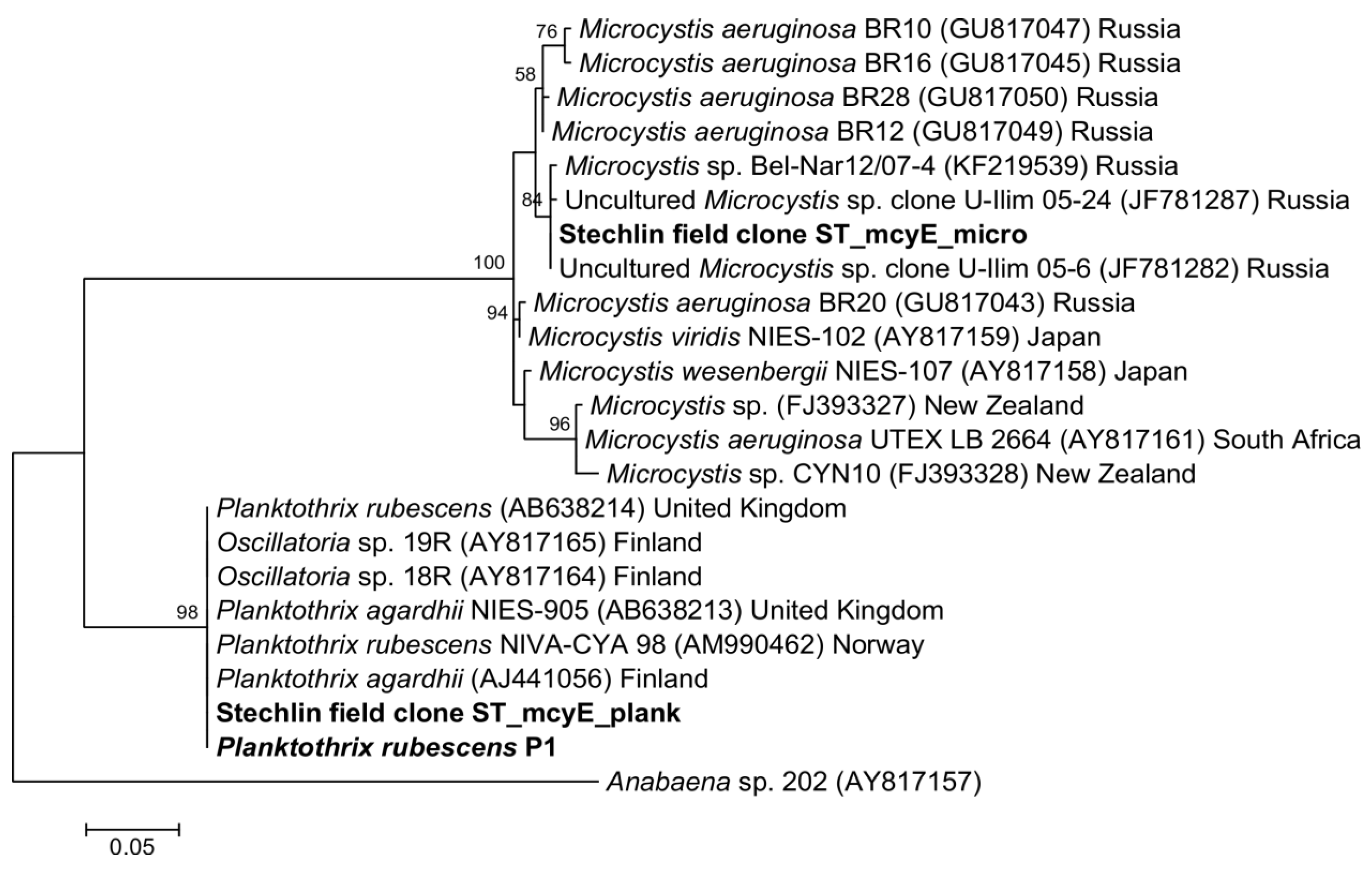
2.2. Molecular Analyses
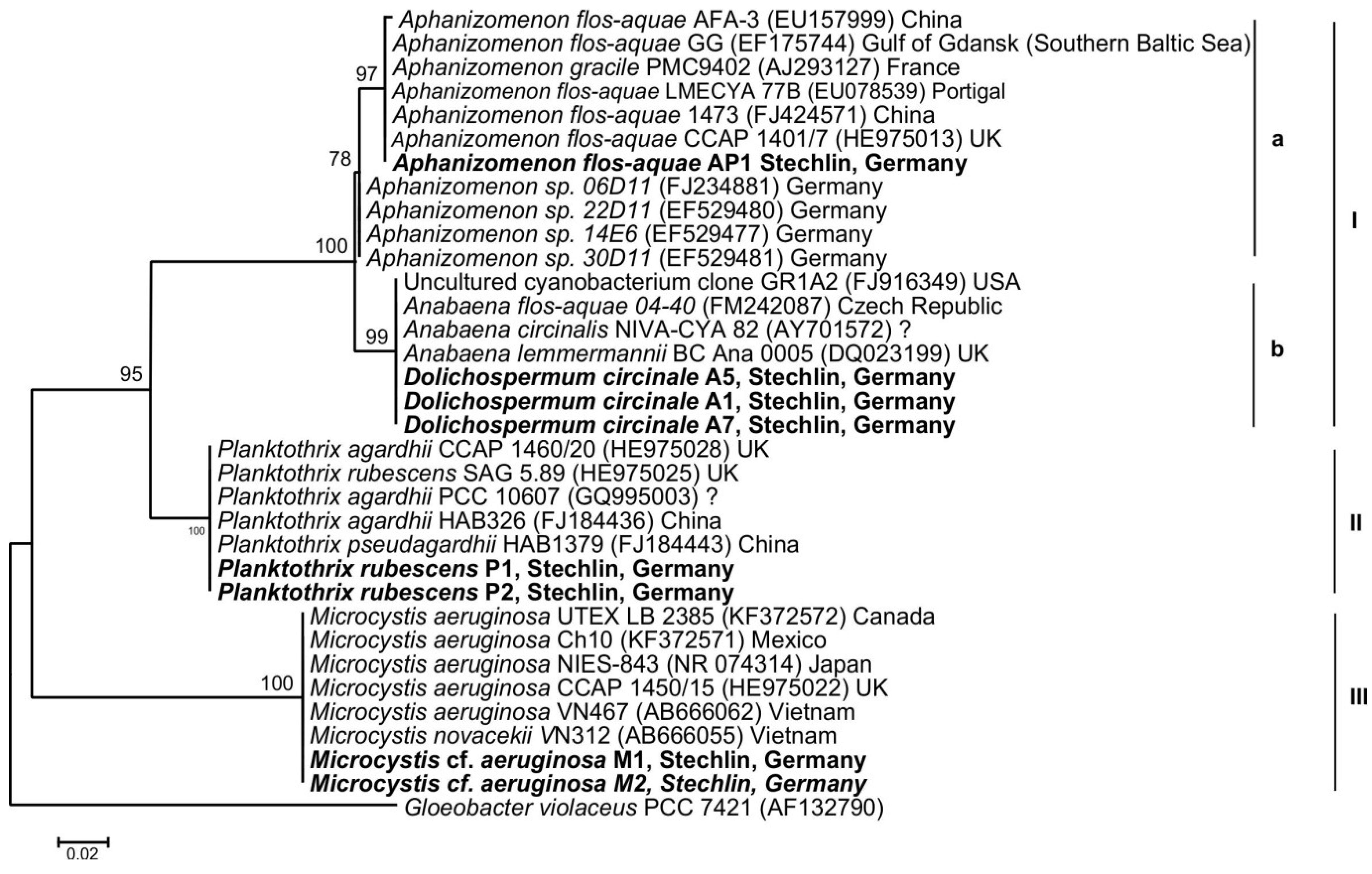
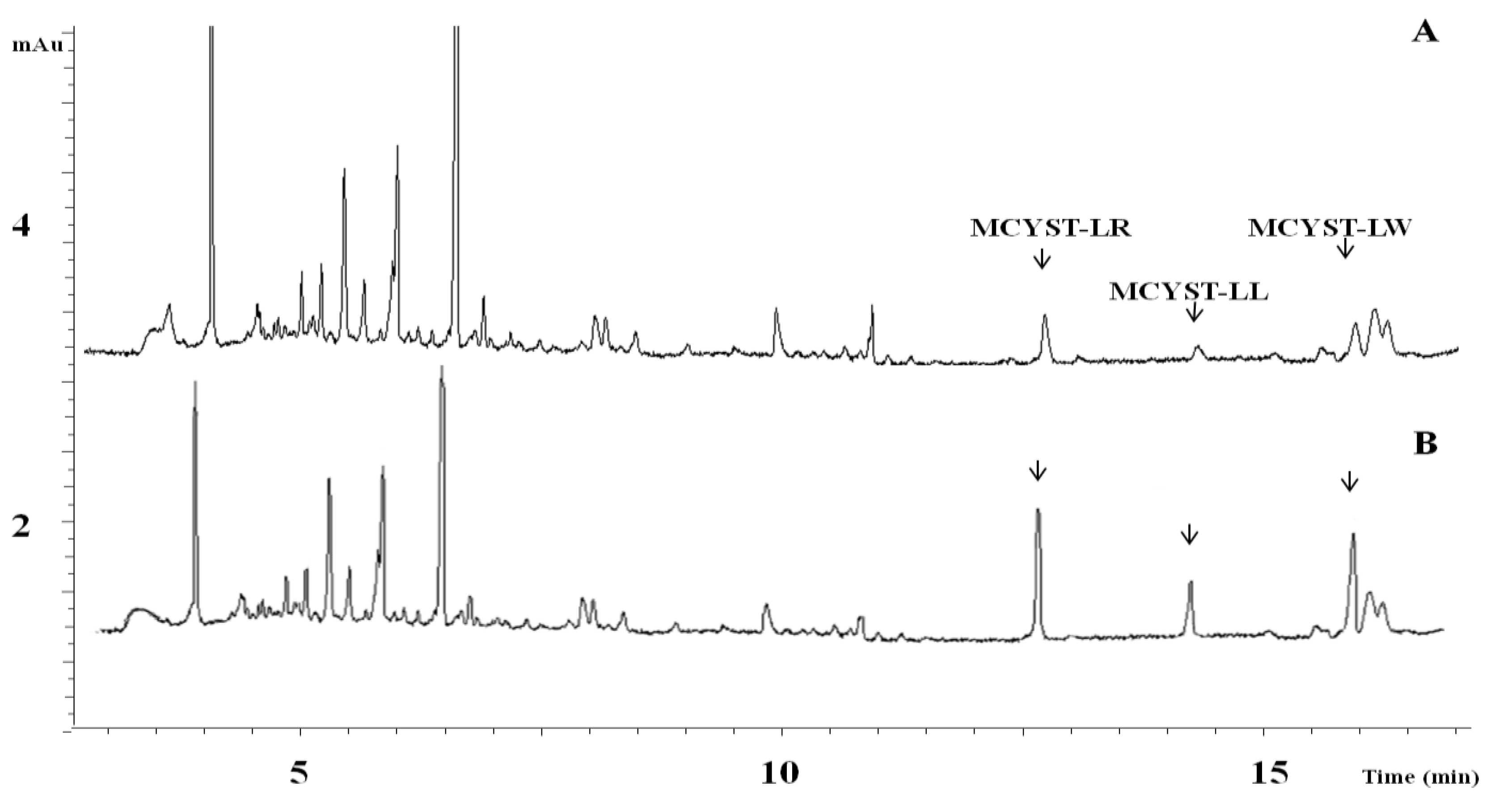
2.3. Cyanotoxin Analyses
| Sample/Strain | Dominant Taxon in Field Sample | Other Taxa in Field Sample | Microcystin Content (µg L−1 LR Equivalent) | Microcystin Variants | Toxin Gene Detected |
|---|---|---|---|---|---|
| July 2011 | Dolichospermum circinale | Microcystis cf. aeruginosa | 27.32 µg L−1 | MCYST-LL, -LR, -LW, -RR | mcyE Microcystis |
| November 2012 | Aphanizomenon flos-aquae | Planktothrix rubescens | 4.25 µg L−1 | MCYST-LW, [Dha7]MCYST-RR | mcyE Planktothrix |
| Dolichospermum circinale A1 | n.d. | n. d. | |||
| Dolichospermum circinale A5 | n.d. | n. d. | |||
| Dolichospermum circinale A7 | n.d. | n. d. | |||
| Aphanizomenon flos-aquae AP1 | n.d. | n. d. | |||
| Microcystis cf. aeruginosa M1 | n.d. | n. d. | |||
| Microcystis cf. aeruginosa M2 | n.d. | n. d. | |||
| Planktothrix rubescens P1 | n.d. | [Dha7]MCYST-RR | mcyE Planktothrix | ||
| Planktothrix rubescens P2 | n.d. | n. d. |
3. Discussion
4. Experimental Section
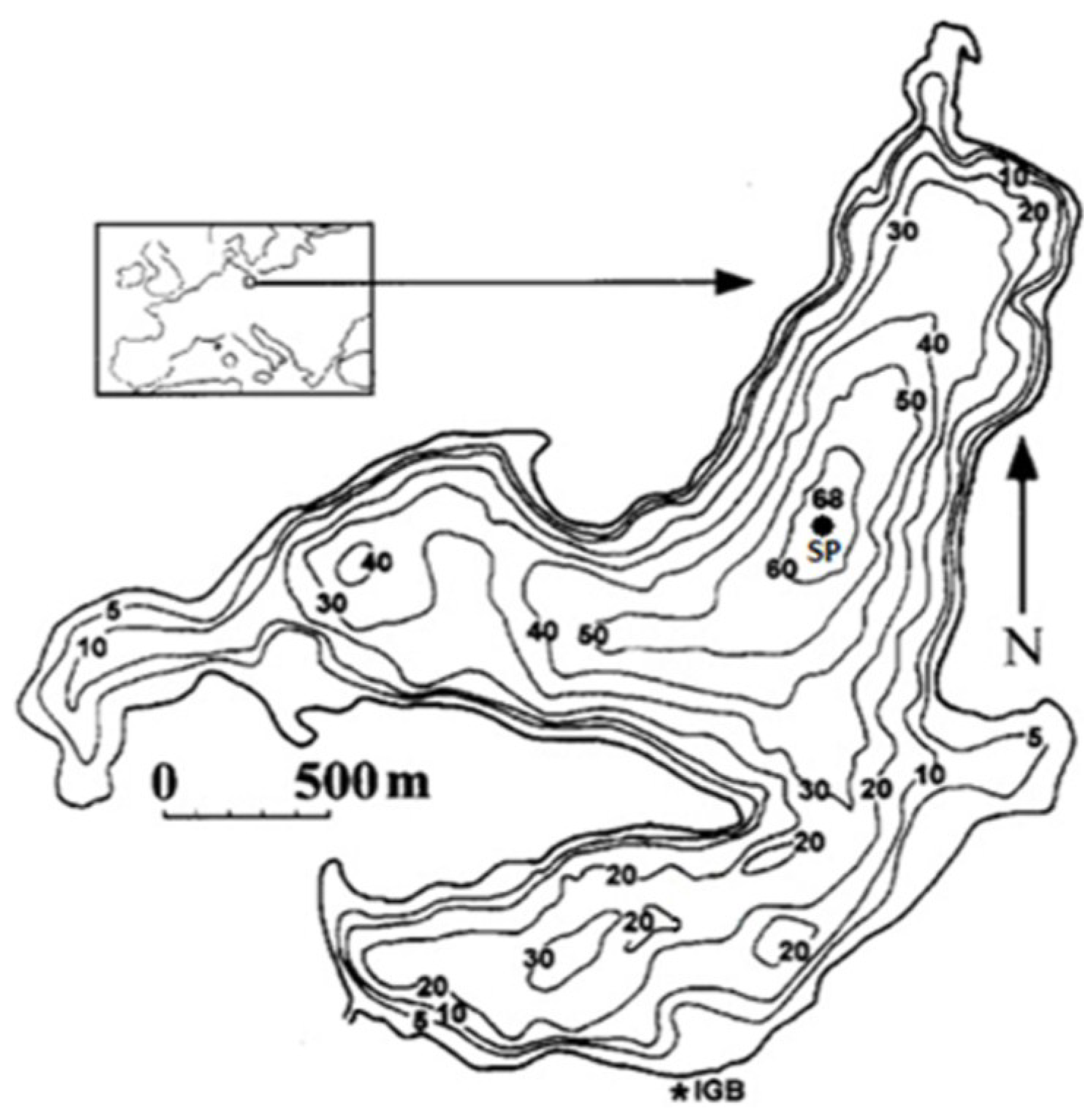
4.1. Site Description and Sampling
4.2. Biomass Calculation, Culturing and Microscopy
4.3. Molecular Analyses
4.4. Toxin Analyses
4.4.1. Quantification of the MCYSTs
4.4.2. MALDI-TOF MS Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casper, S.J. Lake Stechlin: A temperate oligotrophic lake. In Monographiae Biologicae; Dr. W. Junk Publ.: Dordrecht, The Netherland; Boston, MA, USA; Lancaster, UK, 1985; Volume 58, pp. 1–553. [Google Scholar]
- Mathes, J.; Plambeck, G.; Schaumburg, J. Das Typisierungssystem für stehende Gewässer in Deutschland mit Wasserflächen ab 0.5 km2 zur Umsetzung der Wasserrahmenrichtlinie. In Implementierung der EU-Wasserrahmenrichtlinie in Deutschland: Ausgewählte Bewertungsmethoden und Defizite; Deneke, R., Nixdorf, B., Eds.; Brandenburgische Technische Universität: Cottbus, Germany, 2002; pp. 15–23. [Google Scholar]
- Busse, G. Phytoplankton. In Grundlagen zur Modellierungen des Stoffhaushaltes der Gewässer am Beispiel des Stechlin-Sees; Abschlußbericht: Jena, Germany, 1972; pp. 1–44, (unpublished). [Google Scholar]
- Küchler, L. Phytoplanktonuntersuchungen im Stechlinseengebiet in den Jahren 1973–1975. Limnologica 1981, 13, 83–99. [Google Scholar]
- Küchler, L. Phytoplanktonuntersuchungen im Stechlin und im Nordbecken de Nehmitzsees. Limnologica 1982, 14, 231–242. [Google Scholar]
- Padisák, J.; Hajnal, É.; Krienitz, L.; Lakner, J.; Üveges, V. Rarity, ecological memory, rate of floral change in phytoplankton-and the mystery of the Red Cock. Hydrobiologia 2010, 653, 45–64. [Google Scholar]
- Bittencourt-Oliveira, M.D.C. Detection of potential microcystin-producing cyanobacteria in Brazilian reservoirs with a mcyB molecular marker. Harmful Algae 2003, 2, 51–60. [Google Scholar]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.J.; Beattie, K.A.; Metcalf, J.S. Cyanobacterial toxins, exposure routes and human healths. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: “The cyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharm. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar]
- Wiedner, C.; Chorus, I.; Fastner, J. The waterbodies surveyed for cyanotoxins in Germany. In Cyanotoxins-Occurrence, Causes, Consequences; Chorus, I., Ed.; Springer: Berlin, Germany, 2001; pp. 6–21. [Google Scholar]
- Frank, C.A. Microcystin-producing cyanobacteria in recreational waters in southwestern Germany. Environ. Toxicol. 2002, 17, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Hitzfeld, B.; Dietrich, D. Presence of Planktothrix sp. and cyanobacterial toxins in Lake Ammersee, Germany, and their impact on whitefish (Coregonus lavaretus L.). Environ. Toxicol. 2001, 16, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Li, R.H. Cyanobacteria toxins in the Salton Sea. Saline Syst. 2006, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rantala, A.; Rizzi, E.; Castiglioni, B.; de Bellis, G.; Sivonen, K. Identification of hepatotoxin producing cyanobacteria by DNA-chip. Environ. Microbiol. 2008, 10, 653–664. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Mbedi, S.; Welker, M.; Fastner, J.; Wiedner, C. Variability of the microcystin synthetase gene cluster in the genus Planktothrix (Oscillatoriales, Cyanobacteria). FEMS Microbiol. Lett. 2005, 245, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Üveges, V.; Tapolczai, K.; Krienitz, L.; Padisák, J. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 2012, 698, 263–272. [Google Scholar]
- Kwok, R. Fieldwork: The great outdoors. Nature 2013, 503, 301–303. [Google Scholar] [PubMed]
- Kirillin, G.; Shatwell, T.; Kasprzak, P. Consequences of thermal pollution from a nuclear plant on lake temperature and mixing regime. J. Hydrol. 2013, 496, 47–56. [Google Scholar] [CrossRef]
- Padisák, J.; Scheffler, W.; Kasprzak, P.; Koschel, R.; Krienitz, L. Interannual variability in the phytoplankton composition of Lake Stechlin (1994–2000). Arch. Hydrobiol. 58, 101–133.
- Sommer, U.; Gliwicz, M.; Lampert, W.; Duncan, A. The PEG model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [PubMed]
- Baker, P.D.; Bellifemine, D. Environmental influences on akinete germination of Anabaena circinalis and implication for management of cyanobacterial blooms. Hydrobiologia 2000, 427, 65–73. [Google Scholar]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J. Biodiversity-global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Mehnert, G.; Leunert, F.; Cires, S.; Johnk, K.D.; Rücker, J.; Nixdorf, B.; Wiedner, C. Competitiveness of invasive and nativecyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- Vasas, G.; Farkas, O.; Borics, G.; Felföldi, T.; Sramkó, T.; Batta, G.; Bácsi, I.; Gonda, S. Appearance of Planktothrixrubescens bloom with [D-Asp3, Mdha7] MC–RR in gravel pit pond of a shallow lake-dominated area. Toxins 2013, 5, 2434–2455. [Google Scholar] [PubMed]
- Padisák, J.; Barbosa, F.A.R.; Koschel, R.; Krienitz, L. Deep layer cyanoprokaryota maxima are constitutional features of lakes: Examples from temperate and tropical regions. Arch. Hydrobiol. 2003, 58, 175–199. [Google Scholar]
- Rantala, A.; Rajaniemi-Wacklin, P.; Lyra, C.; Lepistö, L.; Rintala, J.; Mankiewicz-Boczek, J.; Sivonen, K. Detection of microcystin-producing cyanobacteria in Finnish lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. Appl. Environ. Microbiol. 2006, 72, 6101–6110. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Neilan, B.A.; Erhard, M.; Döhren, H.V.; Börner, T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 1997, 26, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Vaitomaa, J.; Rantala, A.; Halinen, K.; Rouhiainen, L.; Tallberg, P.; Mokelke, L.; Sivonen, K. Quantitative real-time PCR for determination of microcystinsynthetase E copy numbers for Microcystis and Anabaena in lakes. Appl. Environ. Microbiol. 2003, 69, 7289–7297. [Google Scholar] [CrossRef] [PubMed]
- Jungblut, A.D.; Neilan, B.A. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hodoki, Y.; Ohbayashi, K.; Kobayashi, Y.; Okuda, N.; Nakano, S. Detection and identification of potentially toxic cyanobacteria: Ubiquitous distribution of Microcystis aeruginosa and Cuspidothrix issatschenkoi in Japanese lakes. Harmful Algae 2012, 16, 49–57. [Google Scholar]
- Krienitz, L.; Dadheech, P.K.; Fastner, J.; Kotut, K. The rise of potentially toxin producing cyanobacteria in Lake Naivasha, Great African Rift Valley, Kenya. Harmful Algae 2013, 27, 42–51. [Google Scholar]
- Dadheech, P.K.; Krienitz, L.; Kotut, K.; Ballot, A.; Casper, P. Molecular detection of uncultured cyanobacteria and aminotransferase domains for cyanotoxin production in sediments of different Kenyan lakes. FEMS Microbiol. Ecol. 2009, 68, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.H.; Hunter, P.R.; Codd, G.A. Hazardous freshwater cyanobacteria (blue-green algae). The Lancet. 1993, 341, 1519–1520. [Google Scholar] [CrossRef]
- Fastner, J.; Erhard, M.; Carmichael, W.W.; Sun, F.; Rinehart, K.L.; Rönicke, H.; Chorus, I. Characterization and diversity of microcystins in natural blooms andstrains of the genera Microcystis and Planktothrix from German freshwaters. Arch. Hydrobiol. 1999, 145, 147–163. [Google Scholar]
- World Health Organization (WHO). Guidelines for drinking-water quality. Addendum to Volume 2. In Health Criteria and Other Supporting Information, 2nd ed.; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Ressom, R.; Soong, F.S.; Fitzgerald, J.; Turczynowicz, L.; El Saadi, O.; Roder, D.; Maynard, T.; Falconer, I. Health Effects of Toxic Cyanobacteria (Blue-Green Algae); National Health and Medical Research Council, Australian Government Publishing Service: Canberra, Australia, 1994; p. 108. [Google Scholar]
- Watanabe, M.F.; Oishi, S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [PubMed]
- Van der Westhuizen, A.J.; Eloff, J.N. Effect of temperature and light on the toxicityand growth of the blue-green alga Microcystis aeruginosa. Z. Pflanzenphysiol. 1985, 110, 157–163. [Google Scholar] [CrossRef]
- Sevilla, E.; Martin-Luna, B.; Vela, L.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Microcystin-LR synthesis as response to nitrogen transcriptional analysis of the mcyD gene in Microcystis aeruginosa PCC7806. Ecotoxicology 2010, 19, 1167–1173. [Google Scholar] [PubMed]
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS One 2012, 7, e38757. [Google Scholar] [PubMed]
- Sevilla, E.; Martin-Luna, B.; Vela, L.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806. Environ. Microbiol. 2008, 10, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [PubMed]
- Mühling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008, 2, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Suda, S.; Li, R.; Watanabe, M.; Oyaizu, H.; Matsumoto, S.; Watanabe, M.M. 16S rDNA sequences and phylogenetic analysis of Microcystis strains with and without phycoerythrin. FEMS Microbiol. Lett. 1998, 164, 119–124. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; DelDot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA sequences and evolutionary relationships among toxic andnontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Evol. Microbiol. 1997, 47, 693–697. [Google Scholar]
- Van Gremberghe, I.; Leliaert, F.; Mergeay, J.; Vanormelingen, P.; van der Gucht, K.; Debeer, A.-E.; Lacerot, G.; de Meester, L.; Vyvermanet, W. Lack of phylogeographic structure in the freshwater cyanobacterium Microcystis aeruginosa suggests global dispersal. PLoS One 2011, 6, e19561. [Google Scholar] [PubMed]
- Casper, P.; Koschel, R. Description of Lake Stechlin. Limnologica 1995, 25, 281–284. [Google Scholar]
- Koschel, R.; Adams, D.D. An approach to understanding a temperate oligotrophic lowland lake (Lake Stechlin, Germany). Arch. Hydrobiol. 2003, 58, 1–19. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der quantitative Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Opticount. Software for enumeration of plankton and particles. Available online: http://science.do-mix.de/software_opticount.php (accessed on 7 June 2014).
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research Publication: Oslo, Norway, 1972; B-11/69; pp. 1–5. [Google Scholar]
- Krienitz, L.; Wirth, M. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 2006, 36, 204–210. [Google Scholar]
- Komárková-Legnerová, J.; Eloranta, P. Planktic blue-green algae (Cyanophyta) from central Finland (Jyväskylä region) with special reference to the genus Anabaena. Archiv für Hydrobiologie. Supplementband. Untersuchungen des Elbe-AEstuars 1992, 67, 103–133. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm: Jena, Germany, 1998; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, part 2. Oscillatoriales. In Süsswasser Flora von Mitteleuropa Band 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Gustav Fischer: Jena, Germany, 2005; p. 759. [Google Scholar]
- Komárek, J.; Komárková, J. Diversity of Aphanizomenon-like cyanobacteria. Czech. Phycol. Olomouc. 2006, 6, 1–32. [Google Scholar]
- Castenholz, R.W. Oxygenic photosynthetic bacteria. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Boone, D.R., Castenholz, R.W., Eds.; Springer-Verlag: New York, NY, USA, 2001; pp. 473–600. [Google Scholar]
- Suda, S.; Watanabe, M.M.; Otsuka, S.; Mahakahant, A.; Yongmanitchai, W.; Nopartnaraporn, N.; Liu, Y.; Day, J.G. Taxonomic revision of water bloom–forming species of oscillatorioid cyanobacteria. Int. J. Syst. Evol. Microbiol. 2002, 52, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Komárková, J. Taxonomic review of the cyanoprokaryotic genera Planktothrix and Planktothricoides. Czech. Phycol. Olomouc. 2004, 4, 1–18. [Google Scholar]
- Wacklin, P.; Hoffmann, L.; Komárek, J. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet et Flahault) comb. nova. Fottea 2009, 9, 59–64. [Google Scholar]
- Dadheech, P.K.; Abed, R.M.M.; Mahmoud, H.; Krishna Mohan, M.; Krienitz, L. Polyphasic characterization of cyanobacteria isolated from desert crusts, andthe description of Desertifilum tharense gen. et sp. nov. (Oscillatoriales). Phycologia 2012, 51, 260–270. [Google Scholar]
- Ballot, A.; Fastner, J.; Wiedner, C. First report of anatoxin-a-producing cyanobacterium Aphanizomenonissatschenkoi in northeastern Germany. Toxicon 56, 964–971.
- Al-Tebrineh, J.; Mihali, T.K.; Pomati, F.; Neilan, B.A. Detection of saxitoxin-producing cyanobacteria and Anabaena circinalis in environmental water blooms by quantitative pcr. Appl. Environ. Microbol. 2010, 76, 7836–7842. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Kana, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Hepperle, D. Align, Multisequence Alignment Editor ver. 05/2008 SequentiX—Digital DNA Processing. Klein Raden, Germany. 2008. Available online: http://www.sequentix.de (accessed on 7 June 2014).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Vasas, G.; Gáspár, A.; Páger, C.; Surányi, G.; Máthé, C.; Hamvas, M.M.; Borbely, G. Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis. Electrophoresis 2004, 25, 108–115. [Google Scholar] [PubMed]
- Vasas, G.; Szydlowska, D.; Gáspár, A.; Welker, M.; Trojanowicz, M.; Borbély, G. Determination of microcystins in environmental samples using capillary electrophoresis. J. Biochem. Bioph. Meth. 2006, 66, 87–97. [Google Scholar] [CrossRef]
- Welker, M.; Fastner, J.; Erhard, M.; Döhren, H. Application of MALDIi-TOF MS in cyanotoxin research. Environ. Toxicol. 2002, 17, 367–374. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadheech, P.K.; Selmeczy, G.B.; Vasas, G.; Padisák, J.; Arp, W.; Tapolczai, K.; Casper, P.; Krienitz, L. Presence of Potential Toxin-Producing Cyanobacteria in an Oligo-Mesotrophic Lake in Baltic Lake District, Germany: An Ecological, Genetic and Toxicological Survey. Toxins 2014, 6, 2912-2931. https://doi.org/10.3390/toxins6102912
Dadheech PK, Selmeczy GB, Vasas G, Padisák J, Arp W, Tapolczai K, Casper P, Krienitz L. Presence of Potential Toxin-Producing Cyanobacteria in an Oligo-Mesotrophic Lake in Baltic Lake District, Germany: An Ecological, Genetic and Toxicological Survey. Toxins. 2014; 6(10):2912-2931. https://doi.org/10.3390/toxins6102912
Chicago/Turabian StyleDadheech, Pawan K., Géza B. Selmeczy, Gábor Vasas, Judit Padisák, Wolfgang Arp, Kálmán Tapolczai, Peter Casper, and Lothar Krienitz. 2014. "Presence of Potential Toxin-Producing Cyanobacteria in an Oligo-Mesotrophic Lake in Baltic Lake District, Germany: An Ecological, Genetic and Toxicological Survey" Toxins 6, no. 10: 2912-2931. https://doi.org/10.3390/toxins6102912




