Neuromuscular Activity of Micrurus laticollaris (Squamata: Elapidae) Venom in Vitro
Abstract
:1. Introduction
2. Results and Discussion


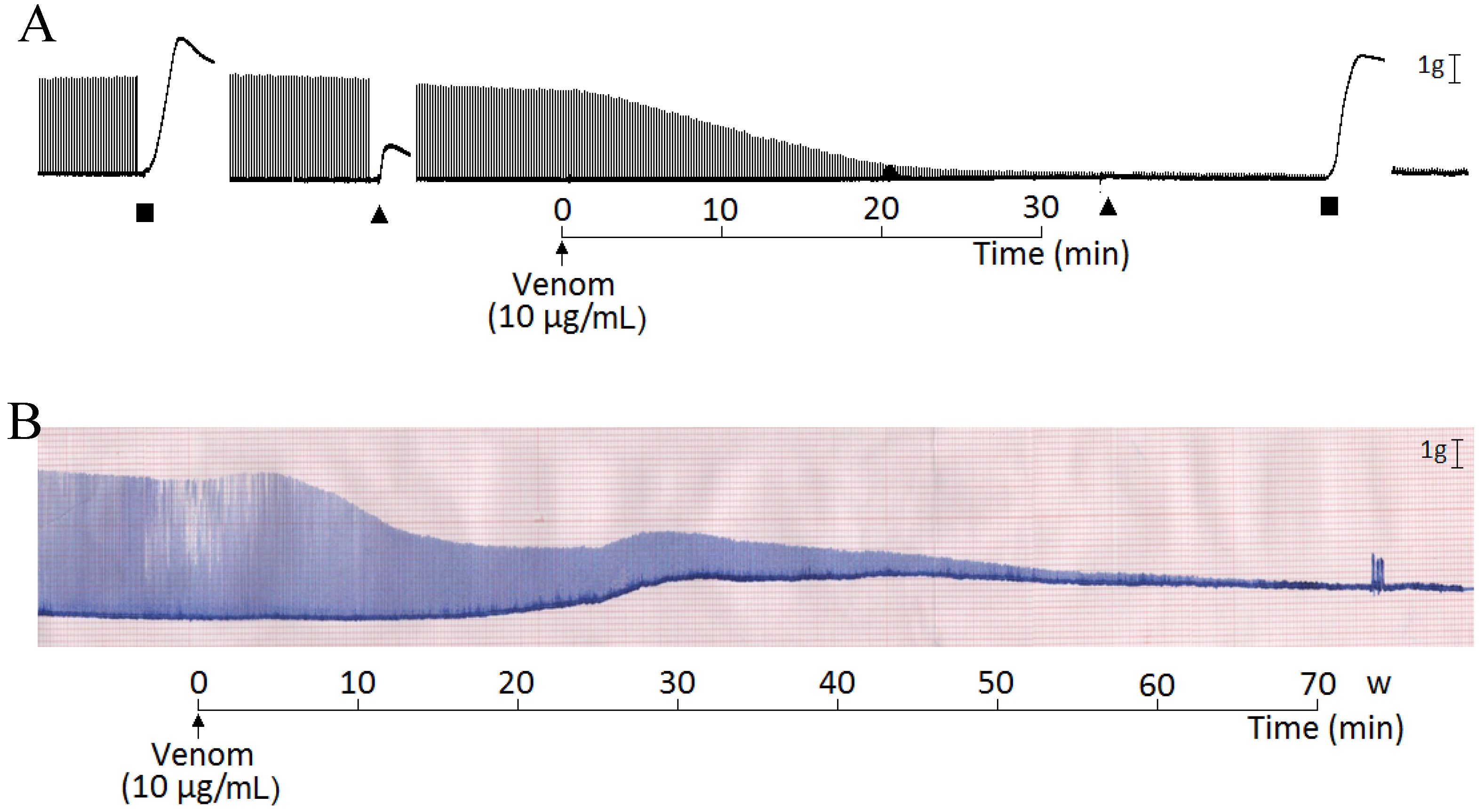
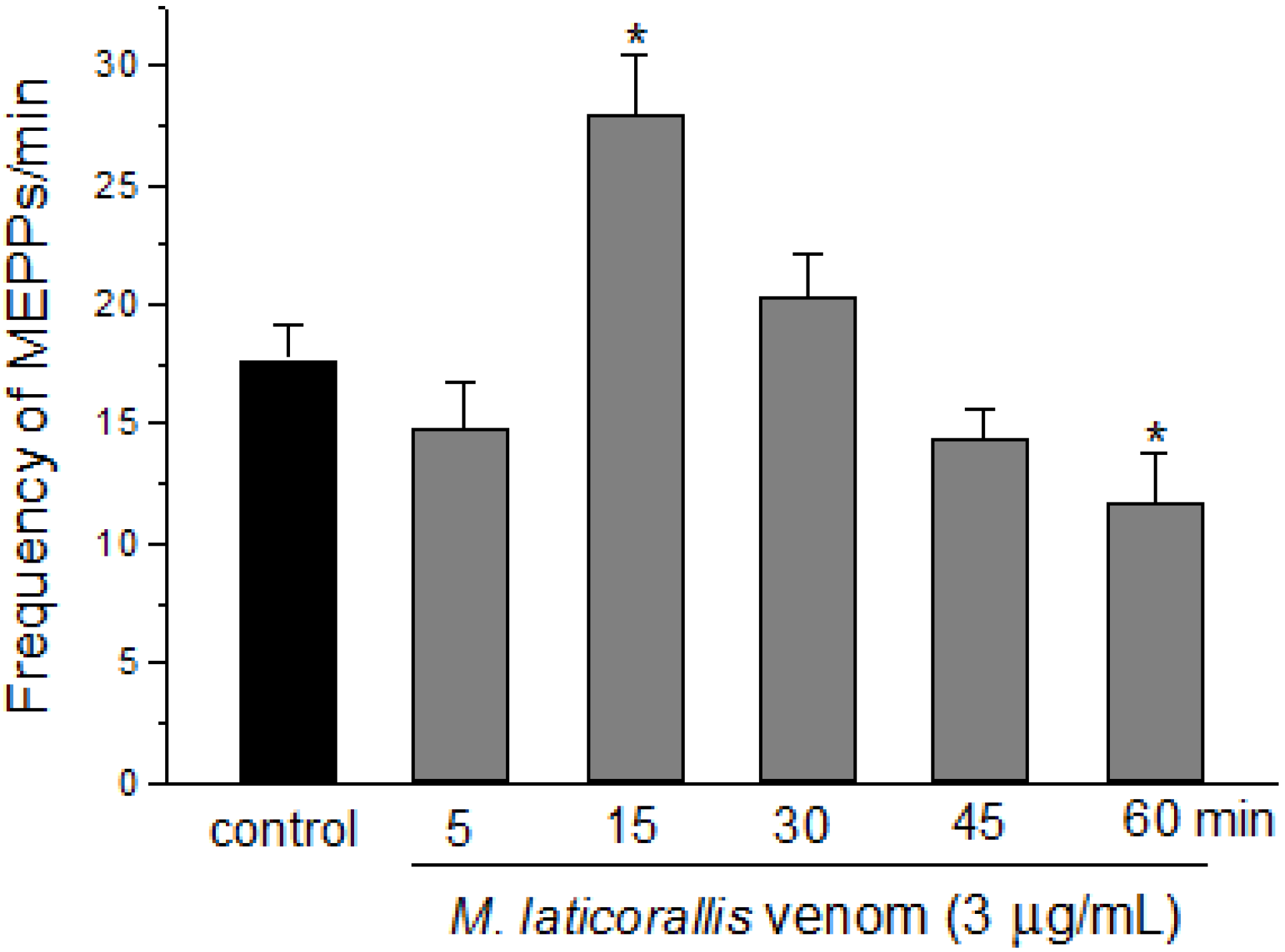
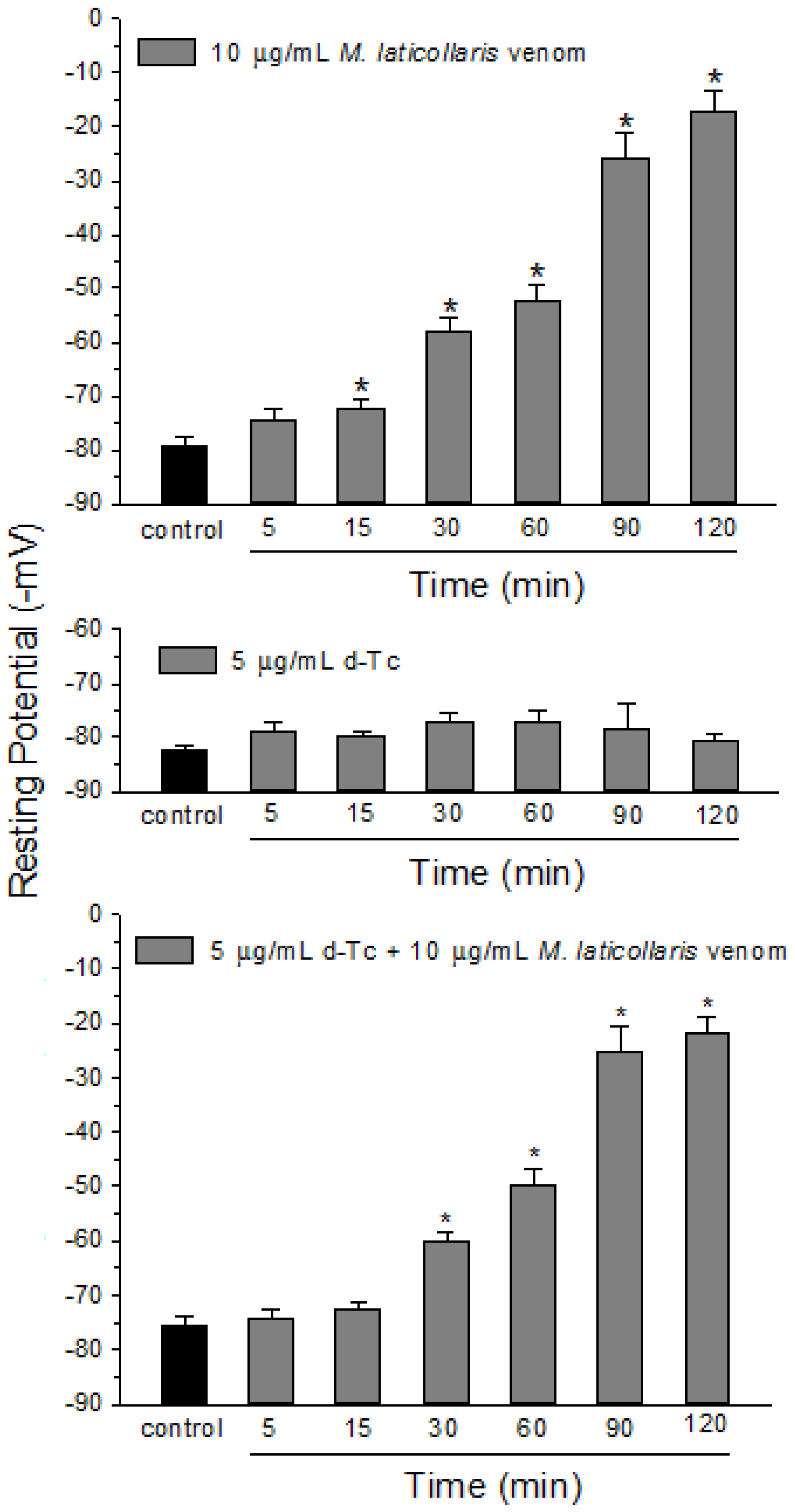
3. Experimental Section
3.1. Venom and Animals
3.2. Twitch-Tension Experiments
3.3. Intracellular Recordings
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Roze, J.A. New World coral snakes (Elapidae): A taxonomic and biological summary. Mem. Inst. Butantan 1982, 46, 305–338. [Google Scholar]
- Roze, J.A. New species and subspecies of coral snakes, genus Micrurus (Elapidae), with notes on type specimens of several species. Am. Mus. Novitates 1989, 2932, 1–15. [Google Scholar]
- Roze, J.A.; Bernal-Carlo, A. Las serpientes corales venenosas del genero Leptomicrurus (serpentes, Elapidae) de Suramérica con descripción de una nueva subespécie. Boll. Mus. Reg. Sci. Nat. Torino 1987, 5, 573–608. [Google Scholar]
- Aird, S.D.; da Silva, N.J., Jr. Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. B 1991, 99, 287–294. [Google Scholar]
- Campbell, J.A.; Lamar, W.W. The Venomous Reptiles of Latin America; Comstock Publishers/Cornell University Press: Ithaca, NY, USA, 1989. [Google Scholar]
- Roze, J.A. Coral Snakes of the Americas. Biology, Identification and Venoms; Struik Publishing Co.: Malabar, FL, USA, 1996. [Google Scholar]
- Vital-Brazil, O. Coral snake venoms: Mode of action and pathophysiology of experimental envenomation. Rev. Inst. Med. Trop. Sao Paulo 1987, 29, 119–126. [Google Scholar] [CrossRef]
- Rosso, J.P.; Vargas-Rosso, O.; Gutiérrez, J.M.; Rochat, H.; Bougis, P.E. Characterization of alpha-neurotoxin and phospholipase A2 activities from Micrurus venoms. Determination of the amino acid sequence and receptor-binding ability of the major alpha-neurotoxin from Micrurus nigrocinctus nigrocinctus. Eur. J. Biochem. 1996, 238, 231–239. [Google Scholar]
- Alape-Girón, A.; Stiles, B.; Schmidt, J.; Girón-Cortes, M.; Thelestam, M.; Jörnvall, H.; Bergman, T. Characterization of multiple nicotinic acetylcholine receptor-binding proteins and phospholipases A2 from the venom of the coral snake Micrurus nigrocinctus. FEBS J. 1996, 380, 29–32. [Google Scholar]
- de Oliveira, J.S.; da Silva, A.R.B.; Soares, M.B.; Stephano, M.A.; Dias, W.O.; Raw, I.; Ho, P.L. Cloning and characterization of an a-neurotoxin- type protein specific for the coral snake Micrurus corallinus. Biochem. Biophys. Res. Comm. 2000, 267, 887–891. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; Portilla, E.; Cerdas, L.; Rojas, E. Local effects induced by coral snake venoms: Evidence of myonecrosis after experimental inoculations of venoms from five species. Toxicon 1983, 21, 777–783. [Google Scholar] [CrossRef]
- Arroyo, O.; Rosso, J.P.; Vargas, O.; Gutiérrez, J.M.; Cerdas, L. Skeletal muscle necrosis induced by a phospholipase A2 isolated from the venom of the coral snake Micrurus nigrocinctus nigrocinctus. Comp. Biochem. Physiol. B 1987, 87, 949–952. [Google Scholar]
- Goularte, F.C.; Cogo, J.C.; Gutiérrez, J.M.; Rodrigues-Simioni, L. The ability of specific antivenom and low temperature to inhibit the myotoxicity and neuromuscular block induced by Micrurus nigrocinctus venom. Toxicon 1995, 33, 679–689. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Persson, B.; Cederlund, E.; Flores-Díaz, M.; Gutiérrez, J.M.; Thelestam, M.; Bergman, T.; Jörnvall, H. Elapid venom toxins: multiple recruitments of ancient scaffolds. Eur. J. Biochem. 1999, 259, 225–234. [Google Scholar] [CrossRef]
- Francis, B.R.; da Silva Júnior, N.J.; Seebart, C.; Casais e Silva, L.L.; Schmidt, J.J.; Kaiser, I.I. Toxins isolated from the venom of the Brazilian coral snake (Micrurus frontalis frontalis) include hemorrhagic type phospholipases A2 and postsynaptic neurotoxins. Toxicon 1997, 35, 1193–1203. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; dos Santos, M.C.; de Furtado, M.F.; de Freitas, M.C.; da Silva, W.D.; Kipnis, T.L. Pro-inflammatory activities in elapid snake venoms. Br. J. Pharmacol. 1994, 112, 723–727. [Google Scholar] [CrossRef]
- Vital-Brazil, O.; Fontana, M.D. Ações pré-juncionais e pós-juncionais da peçonha da cobra coral Micrurus corallinus na junção neuromuscular. Mem. Inst. Butantan 1983/1984, 47/48, 13–26. [Google Scholar]
- Cruz-Höfling, M.A.; Rodrigues-Simioni, L.; Vital-Brazil, O. Ultrastructural changes in neuromuscular junctions of mouse diaphragm caused by the venom of the coral snake Micrurus corallinus. Mem. Inst. Butantan 1983/1984, 47/48, 95–105. [Google Scholar]
- Bolaños, R.; Cerdas, L.; Abalos, J.W. Venoms of coral snakes (Micrurus spp.): Report on a multivalent antivenin for the Americas. Bull. Pan Am. Health Organ. 1978, 12, 23–27. [Google Scholar]
- Carbajal-Saucedo, A.; López-Vera, E.; Bénard-Valle, M.; Smith, E.N.; Zamudio, F.; de Roodt, A.R.; Olvera-Rodríguez, A. Isolation, characterization, cloning and expression of an alpha-neurotoxin from the venom of the Mexican coral snake Micrurus laticollaris (Squamata: Elapidae). Toxicon 2013, 66, 64–74. [Google Scholar] [CrossRef]
- Dal Belo, C.A.; Leite, G.B.; Toyama, M.H.; Marangoni, S.; Corrado, A.P.; Fontana, M.D.; Southan, A.; Rowan, E.G.; Hyslop, S.; Rodrigues-Simioni, L. Pharmacological and structural characterization of a novel phospholipase A2 from Micrurus dumerilii carinicauda venom. Toxicon 2005, 46, 736–750. [Google Scholar] [CrossRef]
- De Abreu, V.A.; Leite, G.B.; Borja-Oliveira, C.; Hyslop, S.; Furtado, M.F.D.; Rodrigues-Simioni, L. Neurotoxicity of Micrurus altirostris (Uruguayan coral snake) venom and its neutralization by commercial coral snake antivenom and specific antiserum raised in rabbits. Clin. Toxicol. 2008, 46, 519–527. [Google Scholar] [CrossRef]
- Camargo, T.M.; Roodt, A.R.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L. The neuromuscular activity of Micrurus pyrrhocryptus venom and its neutralization by commercial and specific coral snake antivenoms. J. Venom Res. 2011, 2, 24–31. [Google Scholar]
- Rey-Suárez, P.; Floriano, R.S.; Rostelato-Ferreira, S.; Saldarriaga-Córdoba, M.; Núñez, V.; Rodrigues-Simioni, L.; Lomonte, B. Mipartoxin-I, a novel three-finger toxin, is the major neurotoxic component in the venom of the redtail coral snake Micrurus mipartitus (Elapide). Toxicon 2012, 60, 851–863. [Google Scholar] [CrossRef]
- Kornhauser, R.; Isbister, G.K.; O’Leary, M.A.; Mirtschin, P.; Dunstan, N.; Hodgson, W.C. Cross-neutralization of the neurotoxic effects of Egyptian cobra venom with commercial tiger snake antivenom. Basic Clin. Pharmacol. Toxicol. 2013, 112, 138–143. [Google Scholar] [CrossRef]
- Ginsborg, B.L.; Warriner, J. The isolated chick biventer cervicis nerve muscle preparation. Br. J. Pharmacol. Chemother. 1960, 15, 410–411. [Google Scholar] [CrossRef]
- Clarke, C.; Kuruppu, S.; Reeve, S.; Ian Smith, A.; Hodgson, W.C. Oxylepitoxin-1, a reversible neurotoxin from the venom of the inland taipan (Oxyuranus microlepidotus). Peptides 2006, 27, 2655–2660. [Google Scholar] [CrossRef]
- Blacklow, B.; Kornhauser, R.; Hains, P.G.; Loiacono, R.; Escoubas, P.; Graudins, A.; Nicholson, G.M. α-Elapitoxin-Aa2a, a long-chain snake α-neurotoxin with potent actions on muscle (α1)(2)βγδ nicotinic receptors, lacks the classical high affinity for neuronal α7 nicotinic receptors. Biochem. Pharmacol. 2011, 81, 314–325. [Google Scholar] [CrossRef]
- Kornhauser, R.; Hart, A.J.; Reeve, S.; Smith, A.I.; Fry, B.G.; Hodgson, W.C. Variations in the pharmacological profile of post-synaptic neurotoxins isolated from the venoms of the Papuan (Oxyuranus scutellatus canni) and coastal (Oxyuranus scutellatus scutellatus) taipans. Neurotoxicology 2010, 31, 239–243. [Google Scholar] [CrossRef]
- Wickramaratna, J.C.; Hodgson, W.C. A pharmacological examination of venoms from three species of death adder (Acanthophis antarcticus, Acanthophis praelongus and Acanthophis pyrrhus). Toxicon 2001, 39, 209–216. [Google Scholar] [CrossRef]
- Young, G.T.; Broad, L.M.; Zwart, R.; Astles, P.C.; Bodkin, M.; Sher, E.; Millar, N.S. Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular β4 amino acids that are implicated in the coupling of binding to channel gating. Mol. Pharmacol. 2007, 71, 389–397. [Google Scholar]
- Toutant, J.P.; Rouaud, T.; le Douarin, G.H. Histochemical properties of the biventer cervicis muscle of the chick: A relationship between multiple innervation and slow-tonic fibre types. Histochem. J. 1981, 13, 481–493. [Google Scholar] [CrossRef]
- Chang, C.C.; Su, M.J.; Tung, L.H. Appearance of new acetylcholine receptors on the baby chick biventer cervicis and denervated rat diaphragm muscles after blockade with α-bungarotoxin. J. Physiol. 1977, 268, 449–465. [Google Scholar]
- Hodgson, W.C.; Wickramaratna, J.C. In vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol. 2002, 29, 807–814. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, T.F.; Chuang, S.T. N,O-di and N,N,O-tri[3H]acetyl α-bungarotoxins as specific labeling agents of cholinergic receptors. Br. J. Pharmacol. 1973, 47, 147–160. [Google Scholar] [CrossRef]
- Chang, C.C.; Su, M.J. Further evidence that extrinsic acetylcholine acts preferentially on extrajunctional receptors in the chick biventer cervicis muscles. Eur. J. Pharmacol. 1975, 33, 337–344. [Google Scholar] [CrossRef]
- Figl, A.; Cohen, B.N.; Quick, M.W.; Davidson, N.; Lester, H.A. Regions of beta 4.beta 2 subunit chimeras that contribute to the agonist selectivity of neuronal nicotinic receptors. FEBS Lett. 1992, 308, 245–248. [Google Scholar] [CrossRef]
- Czajkowski, C.; Kaufmann, C.; Karlin, A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc. Natl. Acad. Sci. USA 1993, 90, 6285–6289. [Google Scholar] [CrossRef]
- Prince, R.J.; Sine, S.M. Molecular dissection of subunit interfaces in the acetylcholine receptor. Identification of residues that determine agonist selectivity. J. Biol. Chem. 1996, 271, 25770–25777. [Google Scholar] [CrossRef]
- Bren, N.; Sine, S.M. Identification of residues in the adult nicotinic acetylcholine receptor that confer selectivity for curariform antagonists. J. Biol. Chem. 1997, 272, 30793–30798. [Google Scholar] [CrossRef]
- Teichert, R.W.; Garcia, C.C.; Potian, J.G.; Schmidt, J.J.; Witzemann, V.; Olivera, B.M.; McArdle, J.J. Peptide-toxin tools for probing the expression and function of fetal and adult subtypes of the nicotinic acetylcholine receptor. Ann. N. Y. Acad. Sci. 2008, 1132, 61–70. [Google Scholar] [CrossRef]
- Serafim, F.G.; Reali, M.; da Cruz-Höfling, M.A.; Fontana, M.D. Action of Micrurus dumerilii carinicauda coral snake venom on the mammalian neuromuscular junction. Toxicon 2002, 40, 167–174. [Google Scholar] [CrossRef]
- Goularte, F.C.; da Cruz-Höfling, M.A.; Corrado, A.P.; Rodrigues-Simioni, L. Electrophysiological and ultrastructural analysis of the neuromuscular blockade and miotoxicity induced by the Micrurus nigrocinctus snake venom. Acta Physiol. Pharmacol. Ther. Latinoam. 1999, 49, 290–296. [Google Scholar]
- Barros, A.C.; Fernandes, D.P.; Ferreira, L.C.; Dos Santos, M.C. Local effects induced by venoms from five species of genus Micrurus sp. (coral snakes). Toxicon 1994, 32, 445–452. [Google Scholar] [CrossRef]
- de Roodt, A.R.; Lago, N.R.; Stock, R.P. Myotoxicity and nephrotoxicity by Micrurus venoms in experimental envenomation. Toxicon 2012, 59, 356–364. [Google Scholar] [CrossRef]
- Fernández, J.; Alape-Girón, A.; Angulo, Y.; Sanz, L.; Gutiérrez, J.M.; Calvete, J.J.; Lomonte, B. Venomic and antivenomic analyses of the Central American coral snake, Micrurus nigrocinctus (Elapidae). J. Proteome Res. 2011, 10, 1816–1827. [Google Scholar] [CrossRef]
- Rodrigues-Simioni, L.; Floriano, R.S.; Rostelato-Ferreira, S.; Sousa, N.C.; Marangoni, S.; Ponce-Soto, L.A.; Carregari, V.C.; Hyslop, S. Presynaptic action of Bothriopsis bilineata smargadina (forest viper) venom in vitro. Toxicon 2011, 58, 140–145. [Google Scholar] [CrossRef]
- Floriano, R.S.; Carregari, V.C.; de Abreu, V.A.; Kenzo-Kagawa, B.; Ponce-Soto, L.A.; da Cruz-Höfling, M.A.; Hyslop, H.; Marangoni, S.; Rodrigues-Simioni, L. Pharmacological study of a new Asp49 phospholipase A2 (Bbil-TX) isolated from Bothriopsis bilineata smargadina (forest viper) venom in vertebrate neuromuscular preparations. Toxicon 2013, 69, 191–199. [Google Scholar] [CrossRef]
- Harvey, A.L.; Barfarz, A.; Thompson, E.; Faiz, A.; Preston, S.; Harris, J.B. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon 1994, 32, 257–265. [Google Scholar] [CrossRef]
- Oshima-Franco, Y.; Leite, G.B.; dal Belo, C.A.; Hyslop, S.; Prado-Franceschi, J.; Cintra, A.C.; Giglio, J.R.; da Cruz-Höfling, M.A.; Rodrigues-Simioni, L. The presynaptic activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom. Basic Clin. Pharmacol. Toxicol. 2004, 95, 175–182. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carbajal-Saucedo, A.; Floriano, R.S.; Belo, C.A.D.; Olvera-Rodríguez, A.; Alagón, A.; Rodrigues-Simioni, L. Neuromuscular Activity of Micrurus laticollaris (Squamata: Elapidae) Venom in Vitro. Toxins 2014, 6, 359-370. https://doi.org/10.3390/toxins6010359
Carbajal-Saucedo A, Floriano RS, Belo CAD, Olvera-Rodríguez A, Alagón A, Rodrigues-Simioni L. Neuromuscular Activity of Micrurus laticollaris (Squamata: Elapidae) Venom in Vitro. Toxins. 2014; 6(1):359-370. https://doi.org/10.3390/toxins6010359
Chicago/Turabian StyleCarbajal-Saucedo, Alejandro, Rafael Stuani Floriano, Cháriston André Dal Belo, Alejandro Olvera-Rodríguez, Alejandro Alagón, and Léa Rodrigues-Simioni. 2014. "Neuromuscular Activity of Micrurus laticollaris (Squamata: Elapidae) Venom in Vitro" Toxins 6, no. 1: 359-370. https://doi.org/10.3390/toxins6010359
APA StyleCarbajal-Saucedo, A., Floriano, R. S., Belo, C. A. D., Olvera-Rodríguez, A., Alagón, A., & Rodrigues-Simioni, L. (2014). Neuromuscular Activity of Micrurus laticollaris (Squamata: Elapidae) Venom in Vitro. Toxins, 6(1), 359-370. https://doi.org/10.3390/toxins6010359



