Expression of VEGF and Flk-1 and Flt-1 Receptors during Blood-Brain Barrier (BBB) Impairment Following Phoneutria nigriventer Spider Venom Exposure
Abstract
:1. Introduction
2. Results
2.1. Blood-Brain Barrier-Associated Proteins: Occludin, β-Catenin, Laminin
2.2. Immunohistochemistry: PNV Increased VEGF, Flt-1, Flk-1 Immunostaining

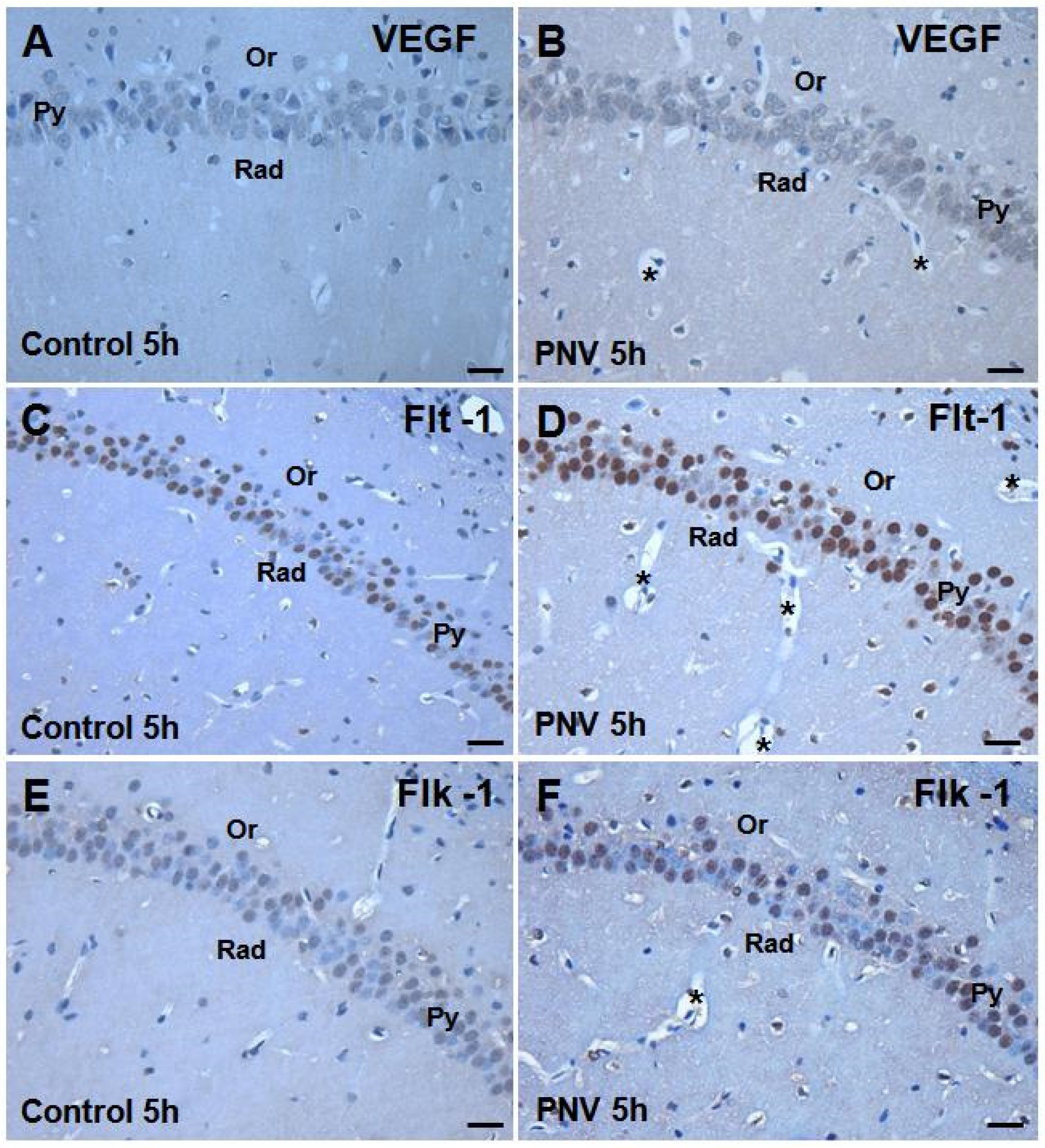
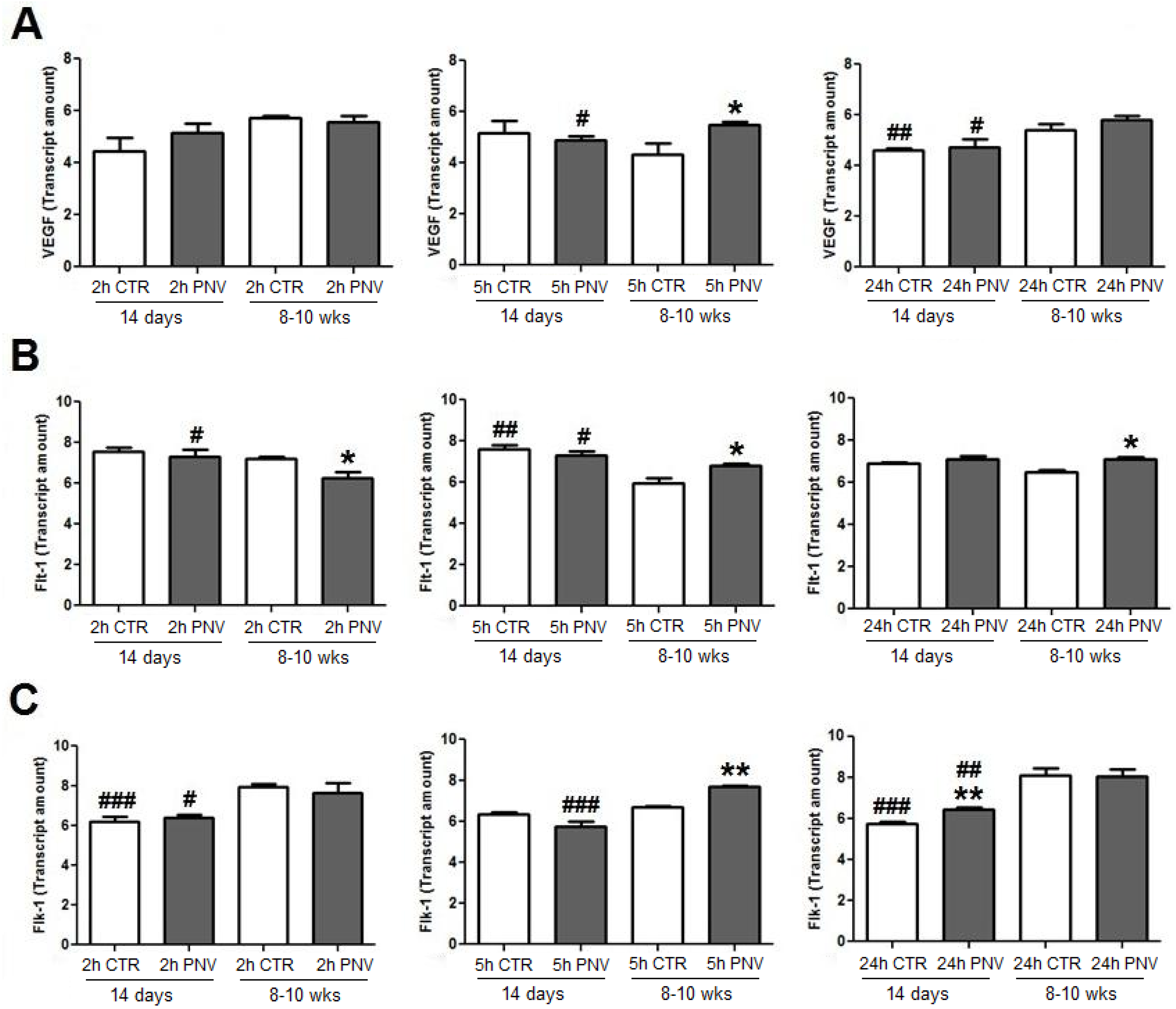
2.3. Western Blotting and Real-Time Polymerase Chain Reaction (PCR): Temporal and Age-Related Differences
2.3.1. VEGF, Flt-1 and Flk-1 Immunoblot Quantification
2.3.2. VEGF, Flt-1 and Flk-1 mRNAs (qPCR)

2.4. NeuN Immunohistochemistry and Western Blotting

3. Discussion
4. Experimental Section
4.1. Animals and Venom
4.2. Envenoming Procedure
4.3. Immunohistochemistry and Image Analysis
4.4. Western Blotting (WB)
4.5. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
4.6. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Vassilevsky, A.A.; Koslov, S.A.; Egorov, T.A.; Grishin, E.V. Purification and characterization of biologically active peptides from spider venoms. Methods Mol. Biol. 2010, 615, 87–100. [Google Scholar] [CrossRef]
- Reis, H.J.; Prado, M.A.; Kalapothakis, E.; Cordeiro, M.N.; Diniz, C.R.; de Marco, L.A.; Gomez, M.V.; Romano-Silva, M.A. Inhibition of glutamate uptake by a polypeptide toxin (phoneutriatoxin 3–4) from the spider Phoneutria nigriventer. Biochem. J. 1999, 343, 413–418. [Google Scholar] [CrossRef]
- Gomez, M.V.; Kalapothakis, E.; Guatimosim, C.; Prado, M.A. Phoneutria nigriventer venom: A cocktail of toxins that affect ion channels. Cell. Mol. Neurobiol. 2002, 22, 579–588. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Deus Reinaldo, C.R.; Hyslop, S.; Madureira, P.R.; de Capitani, E.M.; Vieira, R.J. A clinico-epidemiological study of bites by spiders of the genus Phoneutria. Rev. Inst. Med. Trop. São Paulo 2000, 42, 17–21. [Google Scholar]
- Le Sueur, L.; Kalapothakis, E.; Cruz-Höfling, M.A. Breakdown of the blood-brain barrier and neuropathological changes induced by Phoneutria nigriventer spider venom. Acta Neuropathol. 2003, 105, 125–134. [Google Scholar]
- Le Sueur, L.; Collares-Buzato, C.B.; Cruz-Höfling, M.A. Mechanisms involved in the blood-brain barrier increased permeability induced by Phoneutria nigriventer spider venom in rats. Brain Res. 2004, 1027, 38–47. [Google Scholar] [CrossRef]
- Rapôso, C.; Odorissi, P.A.M.; Oliveira, A.L.R.; Aoyama, H.; Ferreira, C.V.; Verinaud, L.; Fontana, K.; Ruela-de-Sousa, R.R.; da Cruz-Höfling, M.A. Effect of Phoneutria nigriventer venom on the expression of junctional protein and P-gp efflux pump function in the blood-brain barrier. Neurochem. Res. 2012, 37, 1967–1981. [Google Scholar] [CrossRef]
- Nag, S.; Kapadia, A.; Stewart, D.J. Review: Molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol. Appl. Neurobiol. 2011, 37, 3–23. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signaling in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Sköld, M.K.; Risling, M.; Holmin, S. Inhibition of vascular endothelial growth factor receptor 2 activity in experimental brain contusions aggravates injury outcome and leads to early increased neuronal and glial degeneration. Eur. J. Neurosci. 2006, 23, 21–34. [Google Scholar] [CrossRef]
- Ruiz de Almodovar, C.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 2009, 89, 607–648. [Google Scholar] [CrossRef]
- Morin-Brureau, M.; Rigau, V.; Lerner-Natoli, M. Why and how to target angiogenesis in focal epilepsies. Epilepsia 2012, 53, 64–68. [Google Scholar] [CrossRef]
- Rapôso, C.; Zago, G.M.; Silva, G.H.; Cruz-Höfling, M.A. Acute blood brain barrier permeabilization in rats after systemic Phoneutria nigriventer venom. Brain Res. 2007, 1149, 18–29. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Soares, E.S.; Stávale, L.M.; Irazusta, S.P.; Cruz-Höfling, M.A. Upregulation of the vascular endothelial growth factor, Flt-1, in rat hippocampal neurons after envenoming by Phoneutria nigriventer; age-related modulation. Toxicon 2012, 60, 656–664. [Google Scholar] [CrossRef]
- Cruz-Höfling, M.A.; Zago, G.M.; Melo, L.L.; Rapôso, C. C-FOS and n-NOS reactive neurons in response to circulating Phoneutria nigriventer spider venom. Brain Res. Bull. 2007, 73, 114–126. [Google Scholar] [CrossRef]
- Cruz-Höfling, M.A.; Rapôso, C.; Verinaud, L.; Zago, G.M. Neuroinflammationand astrocytic reaction in the course of Phoneutria nigriventer (armed-spider) blood-brain barrier (BBB) opening. Neurotoxicology 2009, 30, 636–646. [Google Scholar] [CrossRef]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; le Couter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Semenza, G. Signal transductions to hypoxia-inducible factor 1. Biochem. Pharmacol. 2002, 64, 993–998. [Google Scholar]
- Góra-Kupilas, K.; Jośko, J. The neuroprotective function of vascular endothelial growth factor (VEGF). Folia Neuropathol. 2005, 43, 31–39. [Google Scholar]
- Zachary, I. Neuroprotective role of vascular endothelial growth factor: Signaling mechanisms, biological function, and therapeutic potential. Neurosignals 2005, 14, 207–221. [Google Scholar] [CrossRef]
- Ogunshola, O.O.; Al-Ahmad, A. HIF-1 at the blood-brain barrier: A mediator of permeability? High Alt. Med. Biol. 2012, 13, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Stávale, L.M.; Soares, E.S.; Mendonça, M.C.; Irazusta, S.P.; da Cruz Höfling, M.A. Temporal relationship between aquaporin-4 and glial fibrillary acidic protein in cerebellum of neonate and adult rats administered a BBB disrupting spider venom. Toxicon 2013, 66, 37–46. [Google Scholar] [CrossRef]
- Risau, W.; Esser, S.; Engelhardt, B. Differentiation of blood-brain barrier endothelial cells. Pathol. Biol. 1998, 46, 171–175. [Google Scholar]
- Witt, K.A.; Mark, K.S.; Hom, S.; Davis, T.P. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2820–H2831. [Google Scholar]
- Nico, B.; Ribatti, D. Morphofunctional aspects of the blood-brain barrier. Curr. Drug Metab. 2012, 13, 50–60. [Google Scholar] [CrossRef]
- Zachary, I.; Gliki, G. Signaling transduction mechanism mediating biological actions of the vascular endothelial growth factor family. Cardiovasc. Res. 2001, 49, 568–581. [Google Scholar] [CrossRef]
- Neuwelt, E.; Abbott, N.J.; Abrey, L.; Banks, W.A.; Blakley, B.; Davis, T.; Engelhardt, B.; Grammas, P.; Nedergaard, M.; Nutt, J.; et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008, 7, 84–96. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar]
- Tillo, M.; Ruhrberg, C.; Mackenzie, F. Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell Adhes. Migr. 2012, 6, 541–546. [Google Scholar] [CrossRef]
- Dent, M.A.; Segura-Araya, E.; Alva-Medina, J.; Aranda-Anzaldo, A. NeuN/Fox-3 is an intrinsic component of the neuronal nuclear matrix. FEBS Lett. 2010, 584, 2767–2771. [Google Scholar] [CrossRef]
- Snyder, J.S.; Ferrante, S.C.; Cameron, H.A. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS One 2012, 7, e48757. [Google Scholar]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [CrossRef]
- Treves, A.; Tashiro, A.; Witter, M.E.; Moser, E.I. What is the mammalian dentate gyrus good for? Neuroscience 2008, 154, 1155–1172. [Google Scholar] [CrossRef]
- Prado, M.A.; Guatimosim, C.; Gomez, M.V.; Diniz, C.; Cordeiro, M.N.; Romano-Silva, M.A. A novel tool for the investigation of glutamate release from rat cerebrocorticalsynaptosomes: The toxin Tx3–3 from the venom of the spider Phoneutria nigriventer. Biochem. J. 1996, 314(Pt 1), 145–150. [Google Scholar]
- Vieira, L.B.; Kushmerick, C.; Reis, H.J.; Diniz, C.R.; Cordeiro, M.N.; Prado, M.A.; Kalapothakis, E.; Romano-Silva, M.A.; Gomez, M.V. PnTx3–6 a spider neurotoxin inhibits K+-evoked increase in Ca2+(i) and Ca2+-dependent glutamate release in synaptosomes. Neurochem. Int. 2003, 42, 277–282. [Google Scholar] [CrossRef]
- Mafra, R.A.; Figueiredo, S.G.; Diniz, C.R.; Cordeiro, M.N.; Cruz, J.D.; de Lima, M.E. PhTx4, a new class of toxins from Phoneutria nigriventer spider venom inhibits the glutamate uptake in rat brain synaptosomes. Brain Res. 1999, 831, 297–300. [Google Scholar] [CrossRef]
- Meissirel, C.; Ruiz de Almodovar, C.; Knevels, E.; Coulon, C.; Chounlamountri, N.; Segura, I.; de Rossi, P.; Vinckier, S.; Anthonis, K.; Deléglise, B.; et al. VEGF modulates NMDA receptors activity in cerebellar granule cells trough Src-family kinases before synapse formation. Proc. Natl. Acad. Sci. USA 2011, 108, 13782–13787. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Li, K.Y.; Huang, Y.L.; Huang, Y.; Sun, F.Y. Vascular endothelial growth factor acutely reduces calcium influx via inhibition of the Ca2+ channels in rat hippocampal neurons. J. Neurosci. Res. 2009, 87, 393–402. [Google Scholar] [CrossRef]
- Bogaert, E.; van Damme, P.; Poesen, K.; Dhondt, J.; Hersmus, N.; Kiraly, D.; Scheveneels, W.; Robberecht, W.; van den Bosch, L. VEGF protects motor neurons against excitotoxicity by upregulation of GluR2. Neurobiology 2010, 31, 2185–2191. [Google Scholar]
- Cammalleri, M.; Martini, D.; Ristori, C.; Timperio, A.M.; Bagnoli, P. Vascular endothelial growth factor up-regulation in the mouse hippocampus and its role in the control of epileptiform activity. Eur. J. Neurosci. 2011, 33, 482–498. [Google Scholar] [CrossRef]
- Mukherjee, S.; Tessema, M.; Wandinger-Ness, A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ. Res. 2006, 98, 743–756. [Google Scholar] [CrossRef]
- Orth, J.D.; McNiven, M.A. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006, 66, 11094–11096. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mendonça, M.C.P.; Soares, E.S.; Stávale, L.M.; Rapôso, C.; Coope, A.; Kalapothakis, E.; Da Cruz-Höfling, M.A. Expression of VEGF and Flk-1 and Flt-1 Receptors during Blood-Brain Barrier (BBB) Impairment Following Phoneutria nigriventer Spider Venom Exposure. Toxins 2013, 5, 2572-2588. https://doi.org/10.3390/toxins5122572
Mendonça MCP, Soares ES, Stávale LM, Rapôso C, Coope A, Kalapothakis E, Da Cruz-Höfling MA. Expression of VEGF and Flk-1 and Flt-1 Receptors during Blood-Brain Barrier (BBB) Impairment Following Phoneutria nigriventer Spider Venom Exposure. Toxins. 2013; 5(12):2572-2588. https://doi.org/10.3390/toxins5122572
Chicago/Turabian StyleMendonça, Monique C. P., Edilene S. Soares, Leila M. Stávale, Catarina Rapôso, Andressa Coope, Evanguedes Kalapothakis, and Maria Alice Da Cruz-Höfling. 2013. "Expression of VEGF and Flk-1 and Flt-1 Receptors during Blood-Brain Barrier (BBB) Impairment Following Phoneutria nigriventer Spider Venom Exposure" Toxins 5, no. 12: 2572-2588. https://doi.org/10.3390/toxins5122572




