Development of Treatment Concepts for the Use of Botulinum Toxin A in Children with Cerebral Palsy
Abstract
:1. Introduction
1.1. Background
1.2. Rationale for BoNT-A Application
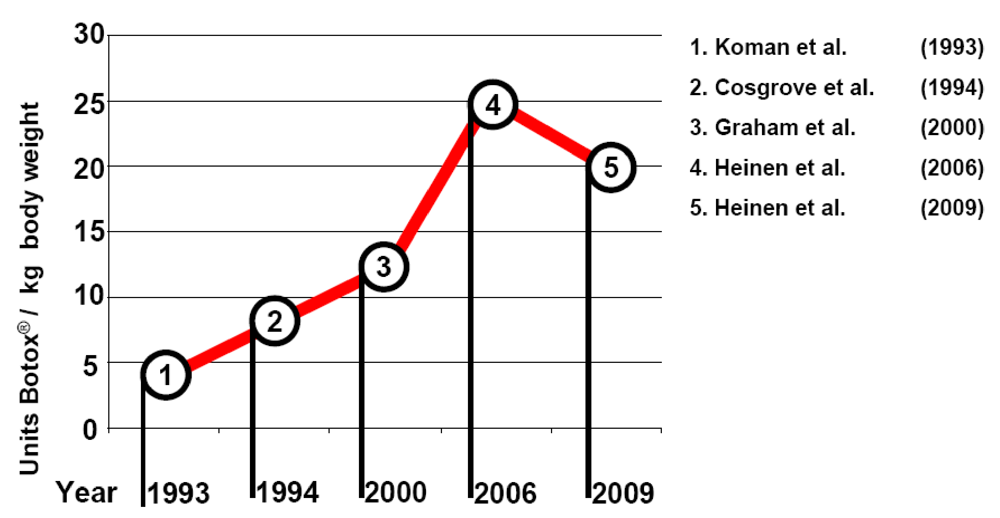
1.3. Safety
2. Previous Development
High-Dose Multi-Level BoNT-A Therapy
3. Key-Muscle Treatment
- Treatment goal: reaching the next motor milestone, the next stage of physiological motor development;
- Selection of the key-muscles;
- Early commencement of treatment;
- Long-term treatment option.
3.1. Treatment Goal: Reaching the Next Motor Milestone

3.2. Selection of the Key-Muscles
3.3. Early Commencement of Treatment
3.4. Long-Term Treatment Option
4. Two Case Reports Exemplifying BoNT-A Therapy According to the Key-Muscle-Concept
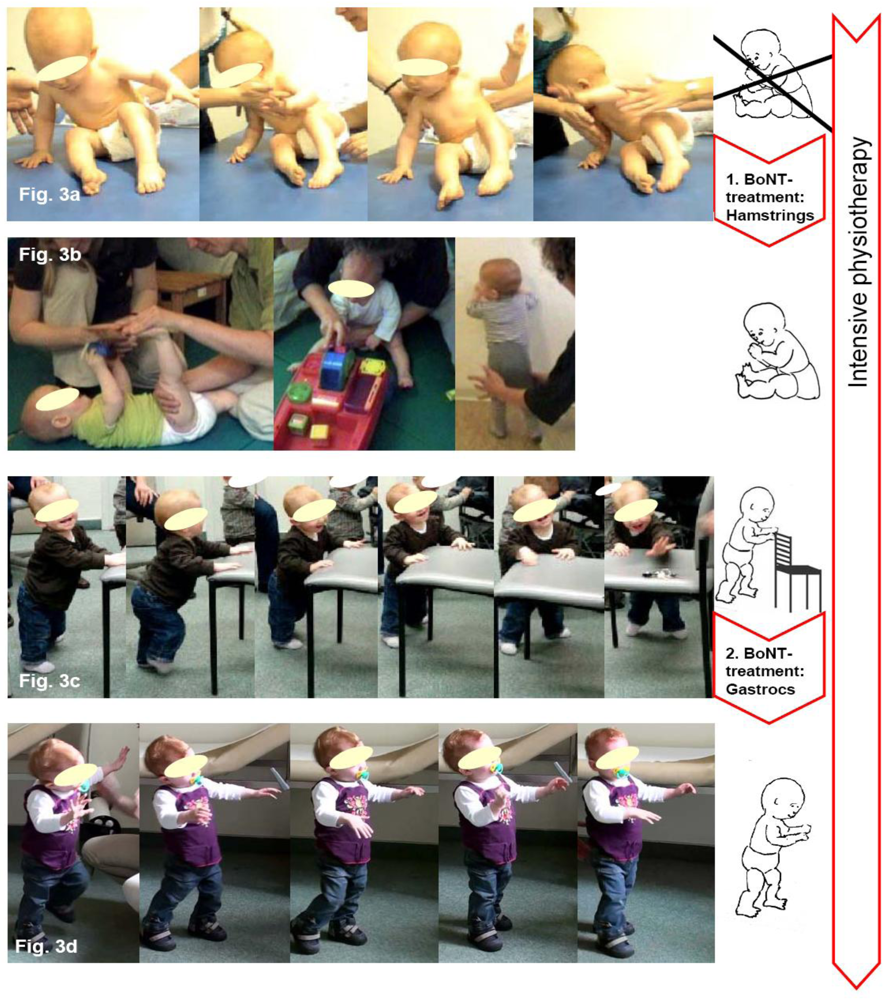
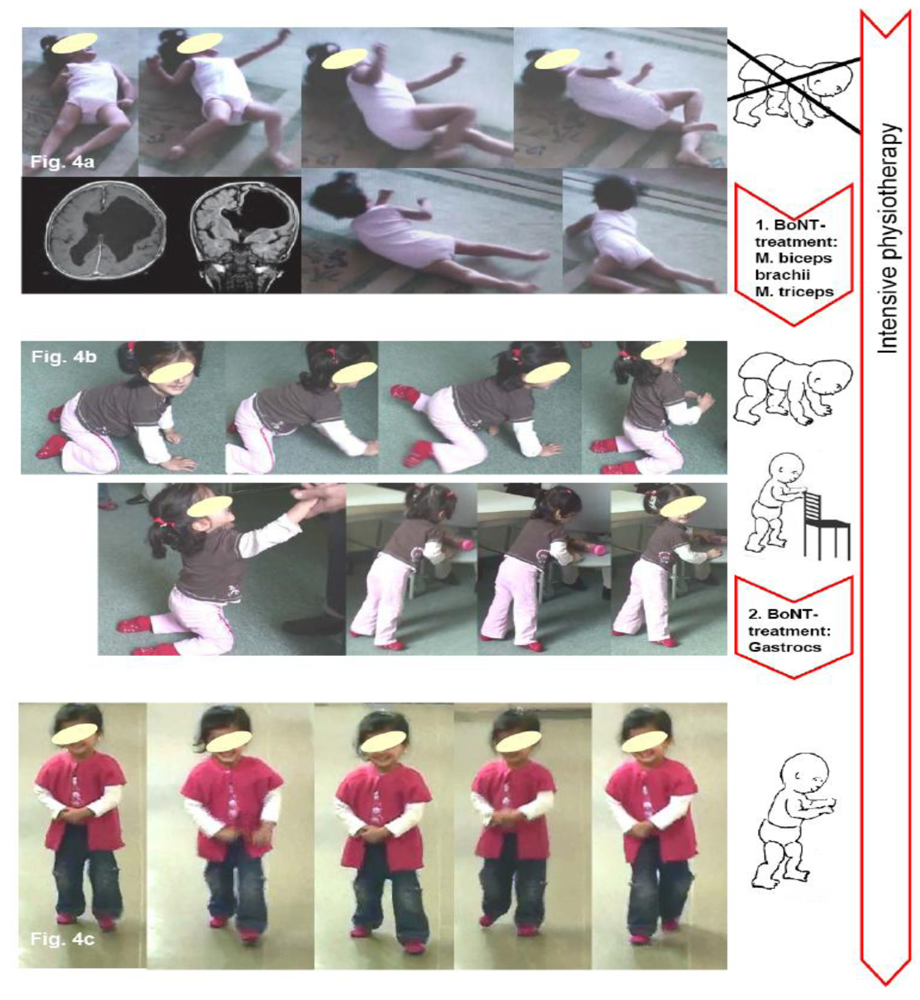
5. Conclusions for Practice
6. Keystones of the Own Procedure in Accordance with the Key-Muscle Concept
6.1. Philosophy
6.2. Treatment Objective
6.3. Technique of Injection
6.4. Injection Solution
6.5. Multimodal Therapy
6.6. Documentation
Acknowledgements
References
- Koman, L.A.; Mooney, J.F., III; Smith, B.; Goodman, A.; Mulvaney, T. Management of cerebral palsy with Botulinum-A toxin: preliminary investigation. J. Pediatr. Orthop. 1993, 13, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Molenaers, G.; Desloovere, K.; Fabry, G.; De Cock, P. The effects of quantitative gait assessment and botulinum toxin a on musculoskeletal surgery in children with cerebral palsy. J. Bone Joint Surg. Am. 2006, 88, 161–170. [Google Scholar]
- Simpson, D.M.; Gracies, J.M.; Graham, H.K.; Miyasaki, J.M.; Naumann, M.; Russman, B.; Simpson, L.L.; So, Y. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology 2008, 70, 1691–1698. [Google Scholar]
- SCPE. Prevalence and characteristics of children with cerebral palsy in Europe. Dev. Med. Child Neurol. 2002, 44, 633–640. [Google Scholar] [PubMed]
- Rosenbaum, P.L.; Gracies, J.M.; Graham, H.K.; Miyasaki, J.M.; Naumann, M.; Russman, B.; Simpson, L.L.; So, Y. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA 2002, 288, 1357–1363. [Google Scholar]
- Crothers, B.; Paine, R.S. The Natural History of Cerebral Palsy; Harvard University: Cambridge, 1959; p. 299. [Google Scholar]
- Berweck, S.; Heinen, F. Cerebralparese. In Blue Book Botulinumtoxin; Berweck, S., Heinen, F., Eds.; Verlag Hans Huber: Bern, Germany, 2008. [Google Scholar]
- Lukban, M.B.; Rosales, R.L.; Dressler, D. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: A summary of evidence. J. Neural. Transm. 2009, 116, 319–331. [Google Scholar]
- Koman, L.A.; Smith, B.P.; Shilt, J.S. Cerebral palsy. Lancet 2004, 363, 1619–1631. [Google Scholar]
- Baker, R.; Jasinski, M.; Maciag-Tymecka, I.; Michalowska-Mrozek, J.; Bonikowski, M.; Carr, L.; MacLean, J.; Lin, J.P.; Lynch, B.; Theologis, T.; et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: A randomized, double-blind, placebo-controlled, dose-ranging study. Dev. Med. Child. Neurol. 2002, 44, 666–675. [Google Scholar] [PubMed]
- Cosgrove, A.P.; Corry, I.S.; Graham, H.K. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev. Med. Child. Neurol. 1994, 36, 386–396. [Google Scholar]
- Graham, H.K.; Aoki, K.R.; Autti-Ramo, I.; Boyd, R.N.; Delgado, M.R.; Gaebler-Spira, D.J.; Gormley, M.E.; Guyer, B.M.; Heinen, F.; Holton, A.F.; et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture 2000, 11, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Molenaers, G.; Fairhurst, C.; Carr, L.J.; Desloovere, K.; Valayer, E.C.; Morel, E.; Papavassiliou, A.S.; Tedroff, K.; Pascual-Pascual, S.I.; et al. European consensus table 2006 on botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2006, 10, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B. Spasticity treatment with botulinum toxins. J. Neural. Transm. 2008, 115, 607–616. [Google Scholar]
- Rosenbaum, P.L.; Palisano, R.J.; Bartlett, D.J.; Galuppi, B.E.; Russell, D.J. Development of the Gross Motor Function Classification System for cerebral palsy. Dev. Med. Child Neurol. 2008, 50, 249–253. [Google Scholar]
- Naidu, K.; Smith, K.; Sheedy, M.; Adair, B.; Yu, X.; Graham, H.K. Systemic adverse events following botulinum toxin A therapy in children with cerebral palsy. Dev. Med. Child Neurol. 2010, 52, 139–144. [Google Scholar]
- US FDA Administration. Early Communication about an Ongoing Safety Review Botox and Botox Cosmetic (Botulinum toxin Type A) and Myobloc (Botulinum toxin Type B). Available online: http://www.fda.gov/cder/whatsnew.htm (Accessed on 10 August 2008).
- Crowner, B.E.; Brunstrom, J.E.; Racette, B.A. Iatrogenic botulism due to therapeutic Botulinum toxin A injection in a pediatric patient. Clin. Neuropharmacol. 2007, 30, 310–313. [Google Scholar]
- Pharmacovigilance, S. Botox und Dysport: Risiko schwerwiegender systemischer UAW bei Kindern mit Zerebralparese. Available online: http://www.swissmedic.ch/suchen/index.html (Accessed on 21 June 2008).
- Graham, H.K. Safety of Botulinum toxin A in cerebral palsy. Toxicon 2008, 51, 1–54. [Google Scholar]
- Wenger, D.R.; Rang, M. The Art and Practice of Children’s Orthopaedics; Raven: New York, NY, USA, 1993. [Google Scholar]
- Hagglund, G.; Wagner, P. Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 150. [Google Scholar]
- Hagglund, G.; Andersson, S.; Duppe, H.; Lauge-Pedersen, H.; Nordmark, E.; Westbom, L. Prevention of dislocation of the hip in children with cerebral palsy. The first ten years of a population-based prevention programme. J. Bone Joint Surg. Br. 2005, 87, 95–101. [Google Scholar] [CrossRef]
- Molenaers, G.; Schorkhuber, V.; Fagard, K.; van Campenhout, A.; de Cat, J.; Pauwels, P.; Ortibus, E.; de Cock, P.; Desloovere, K. Long-term use of botulinum toxin type A in children with cerebral palsy: Treatment consistency. Eur. J. Paediatr. Neurol. 2008, 13, 421–429. [Google Scholar]
- Blackmore, A.M.; Boettcher-Hunt, E.; Jordan, M.; Chan, M.D. A systematic review of the effects of casting on equinus in children with cerebral palsy: An evidence report of the AACPDM. Dev. Med. Child. Neurol. 2007, 49, 781–790. [Google Scholar]
- Placzek, R. Botulinum toxin A in children with infantile cerebral palsy: Indications and treatment concepts. Orthopade 2010, 39, 23–30. [Google Scholar]
- Placzek, R. Botulinumtoxin in Orthopädie und Sportmedizin; Placzek, R., Ed.; UNI-MED Verlag AG: Bremen, Germany, 2006. [Google Scholar]
- Russell, A.; Cotton, E. The Petö System and Its Evolution in Britain; Acorn Foundation: London, UK, 1994. [Google Scholar]
- Russell, D.; Rosenbaum, P.; Gowland, C.; Hardy, S.; Lane, M.; Plews, N.; McGavin, H.; Cadman, D.; Jarvis, S. Gross Motor Function Measure Manual; Gross Motor Measures Group: Toronto, Canada, 1993. [Google Scholar]
- Wijnhoven, T.M.; de Onis, M.; Onyango, A.W.; Wang, T.; Bjoerneboe, G.E.; Bhandari, N.; Lartey, A.; al Rashidi, B. Assessment of gross motor development in the WHO Multicentre Growth Reference Study. Food Nutr. Bull. 2004, 25, S37–S45. [Google Scholar]
- Kargo, W.J.; Nitz, D.A. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J. Neurosci. 2003, 23, 11255–11269. [Google Scholar]
- Hikosaka, O.; Nakamura, K.; Sakai, K.; Nakahara, H. Central mechanisms of motor skill learning. Curr. Opin. Neurobiol. 2002, 12, 217–222. [Google Scholar]
- Maier, M.A.; Armand, J.; Kirkwood, P.A.; Yang, H.W.; Davis, J.N.; Lemon, R.N. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb. Cortex. 2002, 12, 281–296. [Google Scholar]
- Jang, S.H.; Cho, S.H.; Kim, Y.H.; Kwon, Y.H.; Byun, W.M.; Lee, S.J.; Park, S.M.; Chang, C.H. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport 2004, 15, 395–399. [Google Scholar]
- Ward, N.S. Functional reorganization of the cerebral motor system after stroke. Curr. Opin. Neurol. 2004, 17, 725–730. [Google Scholar]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003, 126, 2476–2496. [Google Scholar]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar]
- Pidcock, F.S.; Fish, D.E.; Johnson-Greene, D.; Borras, I.; McGready, J.; Silberstein, C.E. Hip migration percentage in children with cerebral palsy treated with botulinum toxin type A. Arch. Phys. Med. Rehabil. 2005, 86, 431–435. [Google Scholar]
- Pascual-Pascual, S.I.; Pascual-Castroviejo, I. Safety of botulinum toxin type A in children younger than 2 years. Eur. J. Paediatr. Neurol. 2008, 13, 511–515. [Google Scholar]
- Hagglund, G.; Andersson, S.; Duppe, H.; Lauge-Pedersen, H.; Nordmark, E.; Westbom, L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: results of a population-based health care programme and new techniques to reduce spasticity. J. Pediatr. Orthop. B 2005, 14, 269–273. [Google Scholar]
- Siebold, D.; Rickensdorf, S. Neurologische rehabilitation von kindern mit hirnschädigung im ersten und zweiten lebensjahr—Berliner modell. Praxis der Kinder-Reha 2009, 1, 4–10. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Placzek, R.; Siebold, D.; Funk, J.F. Development of Treatment Concepts for the Use of Botulinum Toxin A in Children with Cerebral Palsy. Toxins 2010, 2, 2258-2271. https://doi.org/10.3390/toxins2092258
Placzek R, Siebold D, Funk JF. Development of Treatment Concepts for the Use of Botulinum Toxin A in Children with Cerebral Palsy. Toxins. 2010; 2(9):2258-2271. https://doi.org/10.3390/toxins2092258
Chicago/Turabian StylePlaczek, Richard, Dagmar Siebold, and Julia F. Funk. 2010. "Development of Treatment Concepts for the Use of Botulinum Toxin A in Children with Cerebral Palsy" Toxins 2, no. 9: 2258-2271. https://doi.org/10.3390/toxins2092258



