Investigation of Weather Triggers Preceding Outbreaks of Acute Bovine Liver Disease in Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data
2.2.1. Acute Bovine Liver Disease Cases
2.2.2. Weather Data
2.3. Statistical Analysis
2.3.1. Weather Data Handling and Summary
2.3.2. Data Analysis
3. Results
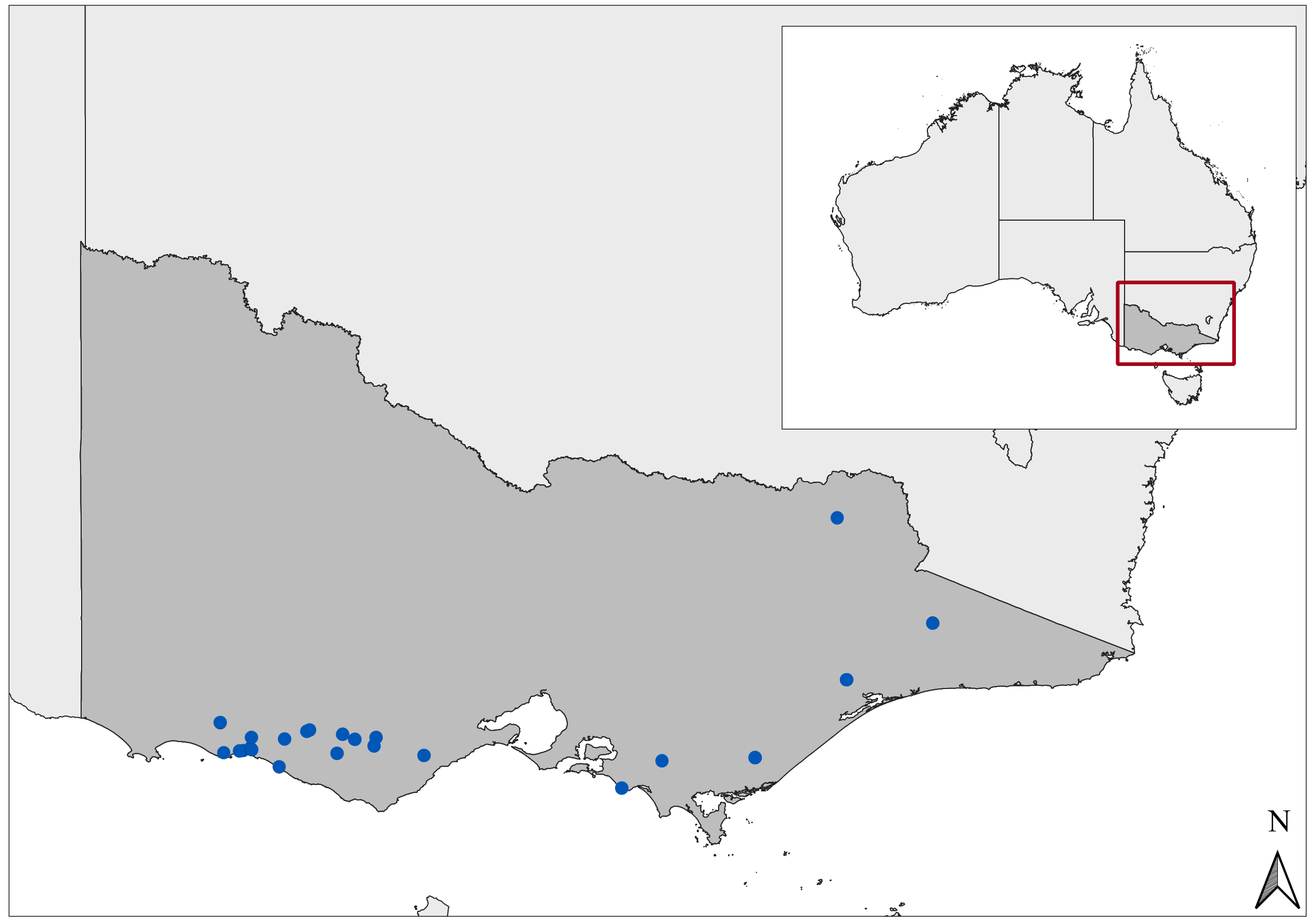
3.1. Case Description
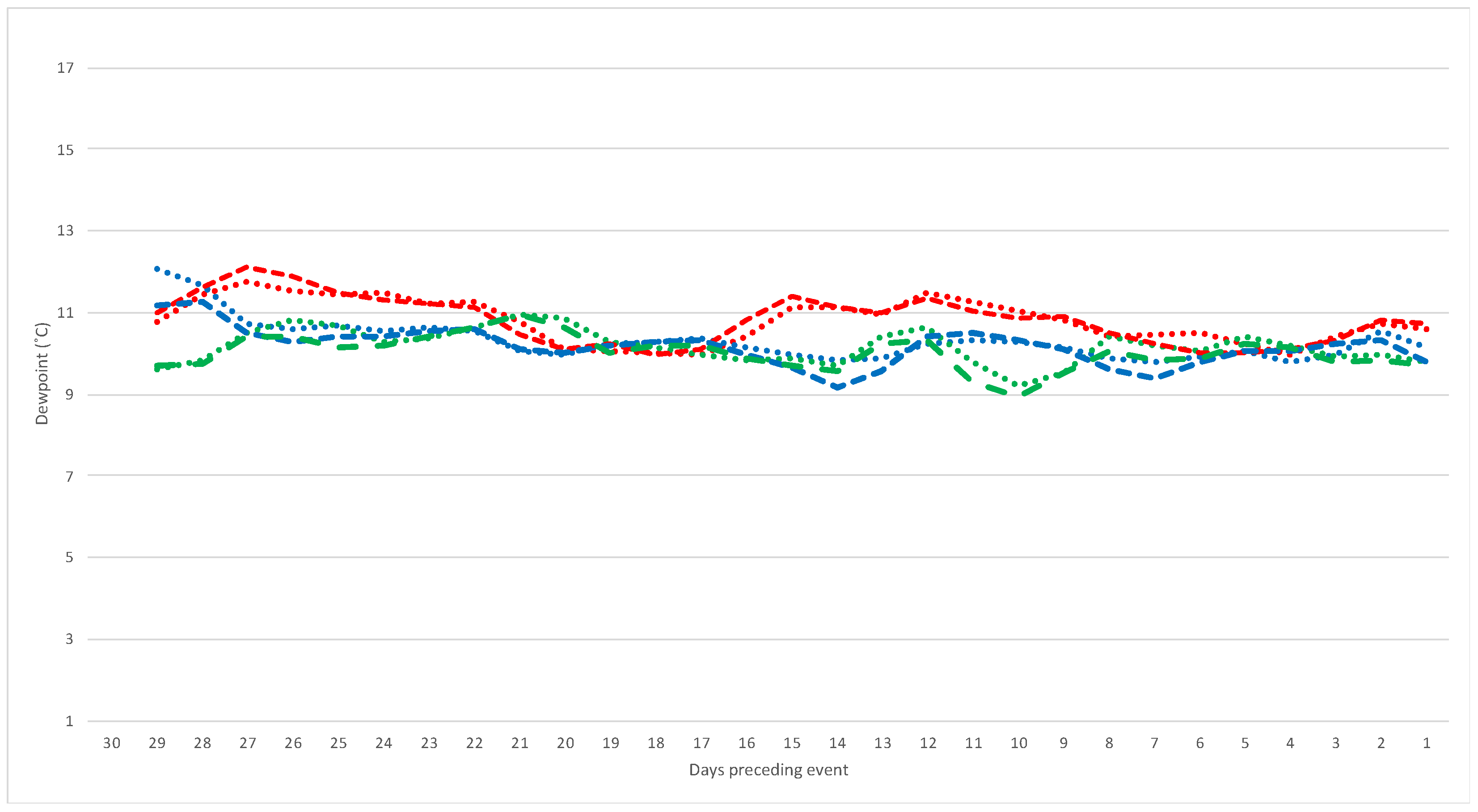
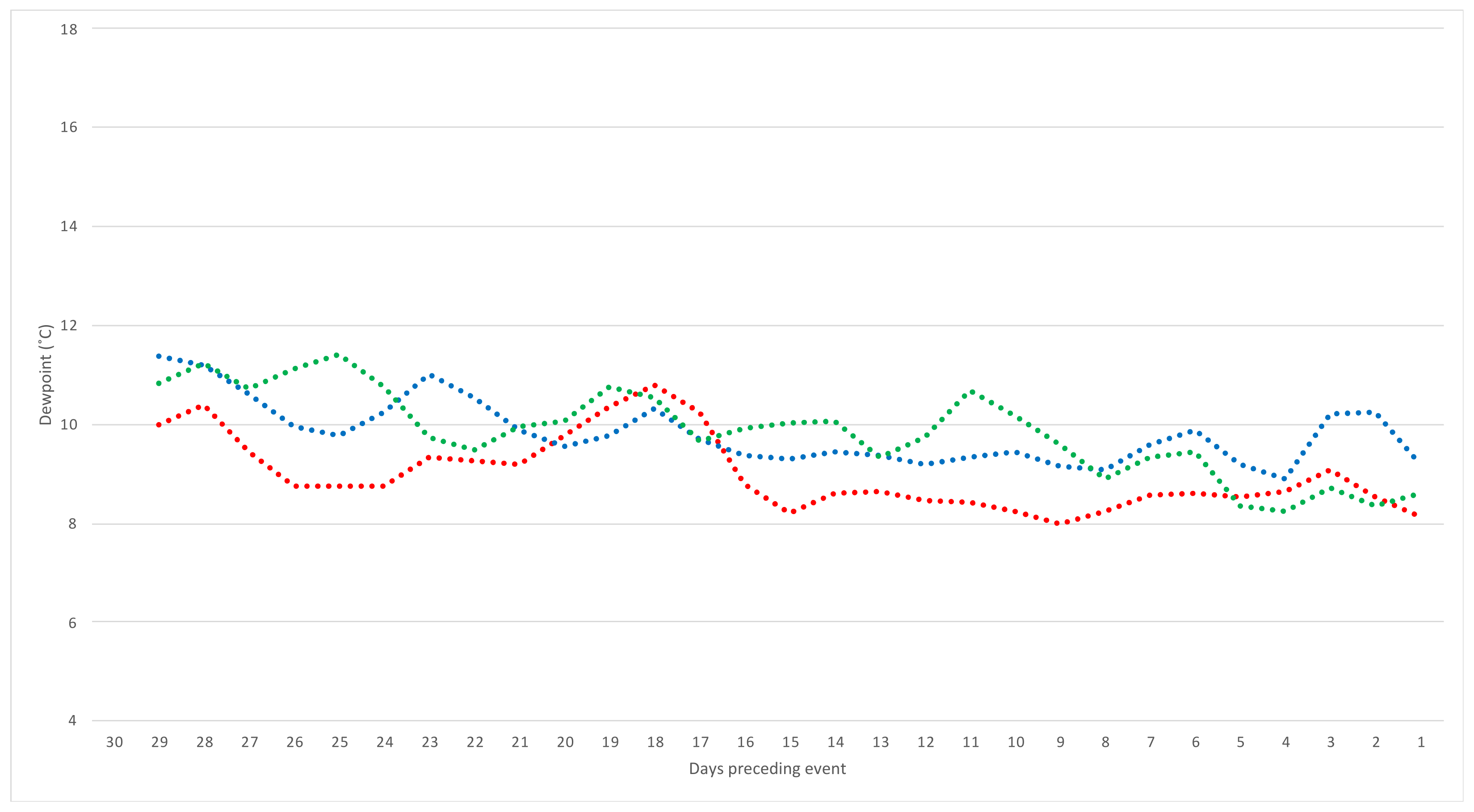
3.2. Weather Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F | female |
| M | male |
| A | adult (>2 years of age) |
| B | biochemistry |
| d | days |
| E | environmental exclusion via paddock and water source examination |
| H | histopathology |
| m | months |
| n/a | not available, details not provided by submitting clinician |
References
- Aslani, M.R.; Pascoe, I.; Kowalski, M.; Michalewicz, A.; Retallick, M.A.S.; Colegate, S.M. In vitro detection of hepatocytotoxic metabolites from Drechslera biseptata: A contributing factor to acute bovine liver disease? Aust. J. Exp. Agric. 2006, 46, 599–604. [Google Scholar] [CrossRef]
- Manthorpe, E.M.; Jerrett, I.V.; Rawlin, G.; Woolford, L. Clinical and pathologic features of acute bovine liver disease in Australia. J. Vet. Diagn. Investig. 2021, 33, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Read, E.; Edwards, J.; Deseo, M.; Rawlin, G.; Rochfort, S. Current Understanding of Acute Bovine Liver Disease in Australia. Toxins 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, M.M.; Woods, L.W.; Poppenga, R.H.; Puschner, B. Amanitin intoxication in two beef calves in California. J. Vet. Diagn. Investig. 2012, 24, 241–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galey, F.D.; Maas, J.; Tronstad, R.J.; Woods, L.W.; Johnson, B.J.; Littlefield, E.S.; Wallstrum, R.; Dorius, L.C. Copper toxicosis in two herds of beef calves following injection with copper disodium edetate. J. Vet. Diagn Investig. 1991, 3, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Stalker, M.J. Hepatic and Biliary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 259–334. [Google Scholar]
- Manthorpe, E.M.; Jerrett, I.V.; Rawlin, G.T.; Woolford, L. Plant and Fungal Hepatotoxicities of Cattle in Australia, with a Focus on Minimally Understood Toxins. Toxins 2020, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Dickason, C. Suspected Acute Bovine Liver Disease. In Animal Health Surveillance Quarterly Report; Animal Health Australia: Canberra, ACT, Australia, 2006; Volume 11, p. 16. [Google Scholar]
- Kelly, W.R.; Gunn, A.; Clarke, R. Acute bovine liver disease (ABLD). In Proceedings of the Annual Meeting, Australian Society for Veterinary Pathology, Sydney, NSW, Australia, 12–13 April 2003; The Veterinary School, University of Sydney: Sydney, NSW, Australia, 2003; pp. 23–25. [Google Scholar]
- Jubb, T. Acute bovine liver disease in cattle. Anim. Health Surveill. 2003, 8, 13. [Google Scholar]
- Lancaster, M.J.; Jubb, T.F.; Pascoe, I.G. Lack of toxicity of rough dog’s tail grass (Cynosurus echinatus) and the fungus Drechslera biseptata for cattle. Aust. Vet. J. 2006, 84, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Bureau of Meterology. Vapour Pressure Map Information. Available online: http://www.bom.gov.au/climate/austmaps/about-vprp-maps.shtml (accessed on 1 March 2022).
- Australian Government Bureau of Meterology. Calculation of Relative Humidity. Available online: http://www.bom.gov.au/climate/averages/climatology/relhum/calc-rh.pdf (accessed on 1 March 2022).
- Australian Government Bureau of Meterology. Feeling Hot and Bothered? It’s Not the Humidity, It’s the Dew Point. Available online: https://media.bom.gov.au/social/blog/1324/feeling-hot-and-bothered-its-not-the-humidity-its-the-dew-point/ (accessed on 1 March 2022).
- Milani, J.L. Ecological conditions affecting mycotoxin production in cereals: A review. Vet. Med. 2013, 58, 405–411. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.S.; Al-Taher, F. Factors Affecting Mycotoxin Production in Fruits. In Mycotoxins in Fruits and Vegetables; Barkai-Golan, R., Paster, N., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 75–104. [Google Scholar] [CrossRef]
- Department of Primary Industries. Parks, Water and Environment. Available online: https://dpipwe.tas.gov.au/biosecurity-tasmania/animal-biosecurity/animal-health/cattle/acute-bovine-liver-disease (accessed on 25 October 2019).
| Property ID | Outbreak ID | Outbreak Date | Season | Morbidity (%, n) | Mortality (%, n) | Signalment | Lactation Status | Diagnostic Method (Number of Individuals Examined) |
|---|---|---|---|---|---|---|---|---|
| Property A | 1 | April 2008 | Autumn | 10 (15/150) | 4 (6/150) | F, A, Holstein | n/a | H (1) |
| Property B | 2 | May 2008 | Autumn | 10 (30/300) | 1 (3/300) | F, A, Holstein | Mid-lactation | H (2) |
| Property C | 3 | April 2010 | Autumn | 22 (11/50) | 2 (1/50) | F, n/a, n/a | n/a | B, E (1) |
| Property D | 4 | April 2010 | Autumn | n/a | n/a | F, n/a, n/a | n/a | H (1) |
| Property E | 5 | April 2010 | Autumn | 25 (50/200) | 1 (3/200) | F, A, Holstein | Mid-lactation | H (10) |
| Property E | 6 | April 2010 | Autumn | 15 (30/200) | 2 (5/200) | F, A, Holstein | Mid-lactation | H (1) |
| Property F | 7 | April 2010 | Autumn | 18 (50/270) | 1 (3/270) | F, A, Holstein | Mid-lactation | H (6) |
| Property G | 8 | March 2011 | Autumn | 10 (20/200) | 1 (2/200) | F, A, Holstein | Mid-lactation | H (2) |
| Property H | 9 | April 2011 | Autumn | 5 (10/200) | 0.5 (1/200) | F, 16 m, Holstein | Mid-lactation | H (1) |
| Property I | 10 | May 2013 | Autumn | 1 (6/400) | 1 (6/400) | F, A, Holstein | Mid-lactation | H (4) |
| Property J | 11 | June 2013 | Winter | 53 (80/150) | 15 (22/150) | F, A, Holstein | Mid-lactation | H (3) |
| Property J | 12 | July 2013 | Autumn | 4 (4/100) | 1 (1/100) | F, A, Holstein | Mid-lactation | H (3) |
| Property K | 13 | April 2014 | Autumn | n/a | 62 (20/32) | F, n/a, Angus | 2–3 d post-weaning | H (1) |
| Property L | 14 | April 2014 | Autumn | 6 (15/250) | 1 (3/250) | F, A, Holstein | Mid-lactation | H (3) |
| Property M | 15 | April 2014 | Autumn | 15 (15/100) | 0 (0/100) | F, A, Angus | Dry | B, E (1) |
| Property N | 16 | April 2014 | Autumn | 5 (7/150) | 0 (0/150) | F, A, Holstein | Mid-lactation | H (5) |
| Property O | 17 | April 2015 | Autumn | 33 (10/30) | 13 (4/30) | F, A, Angus | n/a | H (4) |
| Property P | 18 | November 2017 | Spring | 65 (150/230) | 12 (27/230) | F, A, Holstein | Mid-lactation | H (2) |
| Property Q | 19 | April 2018 | Autumn | 9 (15/160) | 4 (7/160) | F, A, Holstein | Mid-lactation | H (1) |
| Property R | 20 | May 2018 | Autumn | 7 (19/280) | 1 (4/280) | F, A, Holstein | Mid-lactation | H (1) |
| Property S | 21 | May 2018 | Autumn | 4 (30/700) | 0.4 (3/700) | F, A, Holstein x Jersey | n/a | H (1) |
| Property T | 22 | September 2018 | Spring | 0.8 (2/250) | 0 (0/250) | F, A, Holstein | n/a | B, E (2) |
| Property U | 23 | May 2019 | Autumn | 17 (5/30) | 17 (5/30) | F, n/a, n/a | n/a | H (2) |
| Property V | 24 | April 2019 | Autumn | 11 (20/180) | 2 (3/180) | F, A, Holstein | Mid-lactation | B, E (3) |
| Property U | 25 | June 2019 | Winter | n/a | n/a | M, n/a, Angus | n/a | H (1) |
| Property W | 26 | April 2020 | Autumn | 28 (25/88) | 3 (3/88) | F, n/a, n/a | n/a | B, E (5) |
| Weather Parameters | Odds Ratio (95% CI) | p-Value |
|---|---|---|
| Average of daily minimum temperature (°C) | 1.42 (0.98–2.05) | 0.065 |
| Average of daily maximum temperature (°C) | 0.97 (0.71–1.33) | 0.865 |
| Average of daily average temperature (°C) | 1.21 (0.81–1.80) | 0.346 |
| Average of daily temperature gap (°C) | 1.21 (0.81–1.80) | 0.346 |
| Average of daily precipitation (mm) | 1.28 (0.93–1.76) | 0.134 |
| Cumulative daily precipitation (mm) | 1.02 (0.99–1.04) | 0.134 |
| Average of daily solar exposure (MJ/m2) | 0.78 (0.56–1.10) | 0.162 |
| Cumulative daily solar exposure (MJ/m2) | 0.99 (0.96–1.01) | 0.186 |
| Average of daily dewpoint temperature at 9 am (°C) | 2.01 (1.20–3.37) | 0.008 |
| Average of daily dewpoint temperature at 3 pm (°C) | 1.83 (1.15–2.90) | 0.011 |
| Average of daily minimum and dewpoint at 9 am temperature gap (°C) | 0.83 (0.45–1.54) | 0.557 |
| Average of daily maximum and dewpoint at 3 pm temperature gap (°C) | 0.73 (0.54–1.00) | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthorpe, E.M.; Rawlin, G.T.; Stevenson, M.A.; Woolford, L.; Caraguel, C.G.B. Investigation of Weather Triggers Preceding Outbreaks of Acute Bovine Liver Disease in Australia. Toxins 2022, 14, 414. https://doi.org/10.3390/toxins14060414
Manthorpe EM, Rawlin GT, Stevenson MA, Woolford L, Caraguel CGB. Investigation of Weather Triggers Preceding Outbreaks of Acute Bovine Liver Disease in Australia. Toxins. 2022; 14(6):414. https://doi.org/10.3390/toxins14060414
Chicago/Turabian StyleManthorpe, Eve M., Grant T. Rawlin, Mark A. Stevenson, Lucy Woolford, and Charles G. B. Caraguel. 2022. "Investigation of Weather Triggers Preceding Outbreaks of Acute Bovine Liver Disease in Australia" Toxins 14, no. 6: 414. https://doi.org/10.3390/toxins14060414





