Interaction of Clostridium perfringens Epsilon Toxin with the Plasma Membrane: The Role of Amino Acids Y42, Y43 and H162
Abstract
:1. Introduction
2. Results
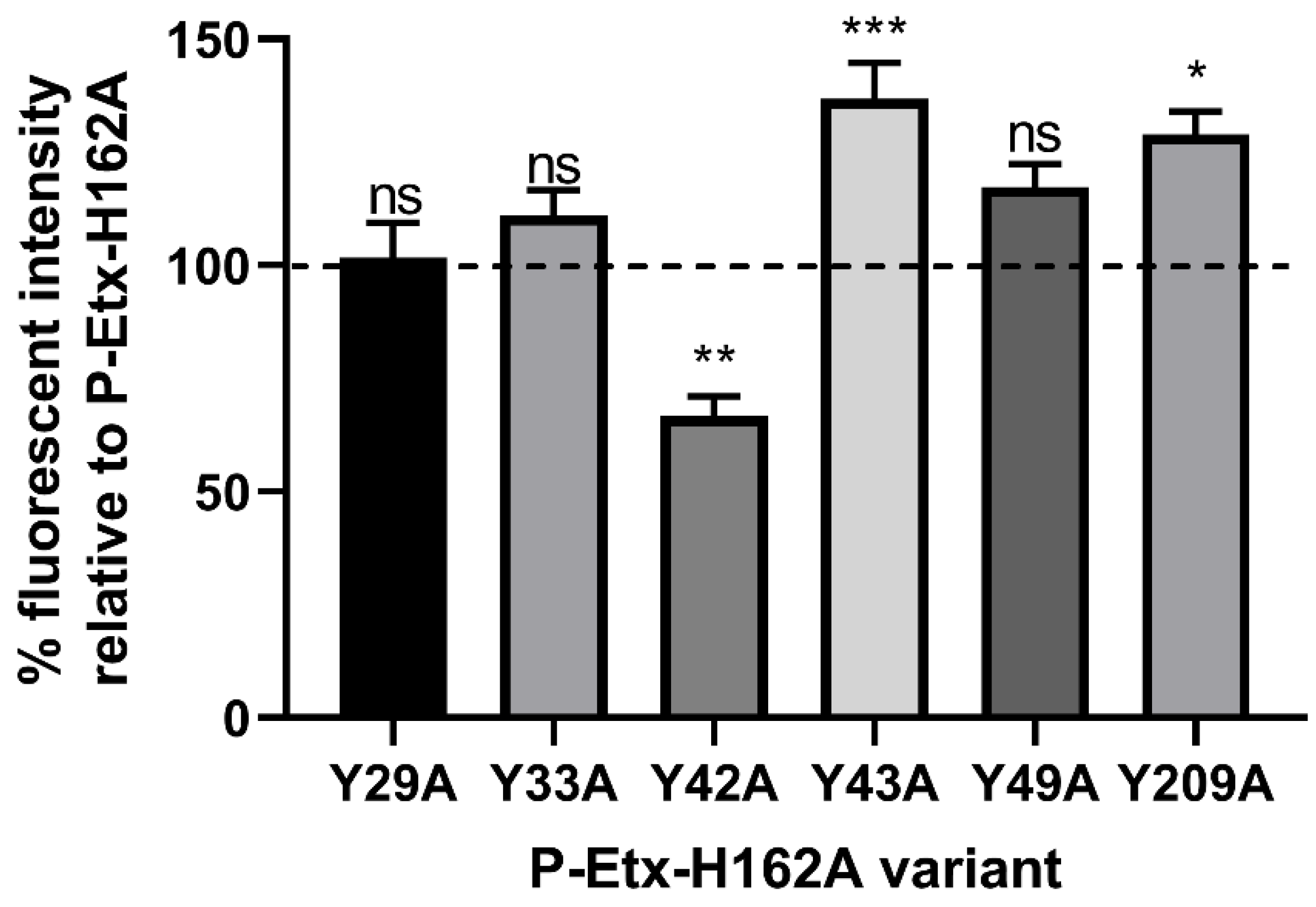
2.1. Surface Exposed Tyrosine Residue Y42 in the RBD Is Critical for P-Etx Binding to CHO-hMAL Cells
2.2. Surface Exposed Tyrosine Residue Y43 Plays a Role in Oligomerisation of Etx in CHO-hMAL Cells
2.3. Etx-H162A Is Inactive towards CHO-hMAL Cells
2.4. Haemolytic Activity of Etx-H162A Is Temperature Dependent, While Haemolytic Activity of Etx-Y42A Is Concentration Dependent
2.5. Mutations Y42A and H162A Affect Oligomerisation of Etx in hRBCs
2.6. Effect of Etx on hRBC Morphology
2.7. Electrophysiology of Etx Pores in Lipid Bilayers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Cell Culture
5.3. Cloning of Recombinant Epsilon Protoxin
5.4. Site-Directed Mutagenesis
5.5. Recombinant Protein Production and Purification
5.6. Activation of P-Etx by Trypsin
5.7. Human Red Blood Cell Ghost Preparation
5.8. On-Cell Western Assay
5.9. Oligomerization Assay
5.10. Cytotoxicity Assay
5.11. Erythrocyte Morphology
5.12. Haemolysis Assay
5.13. Electrophysiology
5.14. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef] [PubMed]
- Rumah, K.R.; Ma, Y.; Linden, J.R.; Oo, M.L.; Anrather, J.; Schaeren-Wiemers, N.; Alonso, M.A.; Fischetti, V.A.; McClain, M.S.; Vartanian, T. The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of Clostridium perfringens epsilon-Toxin. PLoS Pathog. 2015, 11, e1004896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C.; Gnanapavan, S.; Lewis, N.; Popoff, M.R.; Raciborska, D.; Nicholas, R.; et al. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. J. 2019, 25, 653–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrell, T.G.; O’Donoghue, P.J.; Ellis, T. A review of the sheep-multiple sclerosis connection. Med. Hypotheses 1986, 19, 27–39. [Google Scholar] [CrossRef]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.G.; Machado de Avila, R.A.; Chavez-Olortegui, C.D.; Lobato, F.C. Clostridium perfringens epsilon toxin: The third most potent bacterial toxin known. Anaerobe 2014, 30, 102–107. [Google Scholar] [CrossRef]
- Minami, J.; Katayama, S.; Matsushita, O.; Matsushita, C.; Okabe, A. Lambda-toxin of Clostridium perfringens activates the precursor of epsilon-toxin by releasing its N- and C-terminal peptides. Microbiol. Immunol. 1997, 41, 527–535. [Google Scholar] [CrossRef]
- Bhown, A.S.; Habeerb, A.F. Structural studies on epsilon-prototoxin of Clostridium perfringens type D. Localization of the site of tryptic scission necessary for activation to epsilon-toxin. Biochem. Biophys. Res. Commun. 1977, 78, 889–896. [Google Scholar] [CrossRef]
- Miyata, S.; Matsushita, O.; Minami, J.; Katayama, S.; Shimamoto, S.; Okabe, A. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens epsilon-toxin in the synaptosomal membrane. J. Biol. Chem. 2001, 276, 13778–13783. [Google Scholar] [CrossRef] [Green Version]
- Habeeb, A.F.; Lee, C.L.; Atassi, M.Z. Conformational studies on modified proteins and peptides. VII. Conformation of epsilon-prototoxin and epsilon-toxin from Clostridium perfringens. Conformational changes associated with toxicity. Biochim. Biophys. Acta 1973, 322, 245–250. [Google Scholar] [CrossRef]
- Worthington, R.W.; Mulders, M.S. Physical changes in the epsilon prototoxin molecule of Clostridium perfringens during enzymatic activation. Infect. Immun. 1977, 18, 549–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, L.; Maier, E.; Gibert, M.; Popoff, M.R.; Benz, R. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 2001, 276, 15736–15740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortt, S.J.; Titball, R.W.; Lindsay, C.D. An assessment of the in vitro toxicology of Clostridium perfringens type D epsilon-toxin in human and animal cells. Hum. Exp. Toxicol. 2000, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dorca-Arevalo, J.; Dorca, E.; Torrejon-Escribano, B.; Blanch, M.; Martin-Satue, M.; Blasi, J. Lung endothelial cells are sensitive to epsilon toxin from Clostridium perfringens. Vet. Res. 2020, 51, 27. [Google Scholar] [CrossRef] [Green Version]
- Blanch, M.; Dorca-Arevalo, J.; Not, A.; Cases, M.; Gomez de Aranda, I.; Martinez-Yelamos, A.; Martinez-Yelamos, S.; Solsona, C.; Blasi, J. The Cytotoxicity of Epsilon Toxin from Clostridium perfringens on Lymphocytes Is Mediated by MAL Protein Expression. Mol. Cell. Biol. 2018, 38, 00086-18. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xin, W.; Huang, J.; Ji, B.; Gao, S.; Chen, L.; Kang, L.; Yang, H.; Shen, X.; Zhao, B.; et al. Research articleHemolysis in human erythrocytes by Clostridium perfringens epsilon toxin requires activation of P2 receptors. Virulence 2018, 9, 1601–1614. [Google Scholar] [CrossRef] [Green Version]
- Geng, Z.; Huang, J.; Kang, L.; Gao, S.; Yuan, Y.; Li, Y.; Wang, J.; Xin, W.; Wang, J. Clostridium perfringens epsilon toxin binds to erythrocyte MAL receptors and triggers phosphatidylserine exposure. J. Cell. Mol. Med. 2020, 24, 7341–7352. [Google Scholar] [CrossRef]
- Dorca-Arevalo, J.; Blanch, M.; Pradas, M.; Blasi, J. Epsilon toxin from Clostridium perfringens induces cytotoxicity in FRT thyroid epithelial cells. Anaerobe 2018, 53, 43–49. [Google Scholar] [CrossRef]
- Linden, J.R.; Ma, Y.; Zhao, B.; Harris, J.M.; Rumah, K.R.; Schaeren-Wiemers, N.; Vartanian, T. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. mBio 2015, 6, e02513. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, C.D. Assessment of aspects of the toxicity of Clostridium perfringens epsilon-toxin using the MDCK cell line. Hum. Exp. Toxicol. 1996, 15, 904–908. [Google Scholar] [CrossRef]
- Lindsay, C.D.; Hambrook, J.L.; Upshall, D.G. Examination of toxicity of Clostridium perfringens -toxin in the MDCK cell line. Toxicol. Vitr. 1995, 9, 213–218. [Google Scholar] [CrossRef]
- Petit, L.; Gibert, M.; Gillet, D.; Laurent-Winter, C.; Boquet, P.; Popoff, M.R. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J. Bacteriol. 1997, 179, 6480–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, M.; Ochi, S.; Sakurai, J. Assembly of Clostridium perfringens epsilon-toxin on MDCK cell membrane. J. Nat. Toxins 1998, 7, 291–302. [Google Scholar] [PubMed]
- Morcrette, H.; Bokori-Brown, M.; Ong, S.; Bennett, L.; Wren, B.W.; Lewis, N.; Titball, R.W. Clostridium perfringens epsilon toxin vaccine candidate lacking toxicity to cells expressing myelin and lymphocyte protein. NPJ Vaccines 2019, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.A.; Weissman, S.M. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T-cell differentiation. Proc. Natl. Acad. Sci. USA 1987, 84, 1997–2001. [Google Scholar] [CrossRef] [Green Version]
- Rancano, C.; Rubio, T.; Alonso, M.A. Alternative splicing of human T-cell-specific MAL mRNA and its correlation with the exon/intron organization of the gene. Genomics 1994, 21, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Rancano, C.; Rubio, T.; Correas, I.; Alonso, M.A. Genomic structure and subcellular localization of MAL, a human T-cell-specific proteolipid protein. J. Biol. Chem. 1994, 269, 8159–8164. [Google Scholar] [CrossRef]
- Adler, D.; Linden, J.R.; Shetty, S.V.; Ma, Y.; Bokori-Brown, M.; Titball, R.W.; Vartanian, T. Clostridium perfringens Epsilon Toxin Compromises the Blood-Brain Barrier in a Humanized Zebrafish Model. iScience 2019, 15, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Linden, J.R.; Flores, C.; Schmidt, E.F.; Uzal, F.A.; Michel, A.O.; Valenzuela, M.; Dobrow, S.; Vartanian, T. Clostridium perfringens epsilon toxin induces blood brain barrier permeability via caveolae-dependent transcytosis and requires expression of MAL. PLoS Pathog. 2019, 15, e1008014. [Google Scholar] [CrossRef] [Green Version]
- Ivie, S.E.; Fennessey, C.M.; Sheng, J.; Rubin, D.H.; McClain, M.S. Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens epsilon-toxin. PLoS ONE 2011, 6, e17787. [Google Scholar] [CrossRef]
- Gil, C.; Dorca-Arevalo, J.; Blasi, J. Clostridium perfringens Epsilon Toxin Binds to Membrane Lipids and Its Cytotoxic Action Depends on Sulfatide. PLoS ONE 2015, 10, e0140321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahama, M.; Hara, H.; Fernandez-Miyakawa, M.; Itohayashi, Y.; Sakurai, J. Oligomerization of Clostridium perfringens epsilon-toxin is dependent upon membrane fluidity in liposomes. Biochemistry 2006, 45, 296–302. [Google Scholar] [CrossRef]
- Nestorovich, E.M.; Karginov, V.A.; Bezrukov, S.M. Polymer partitioning and ion selectivity suggest asymmetrical shape for the membrane pore formed by epsilon toxin. Biophys. J. 2010, 99, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczesny, P.; Iacovache, I.; Muszewska, A.; Ginalski, K.; van der Goot, F.G.; Grynberg, M. Extending the aerolysin family: From bacteria to vertebrates. PLoS ONE 2011, 6, e20349. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.M.; Abbott, S.L. Evolving concepts regarding the genus Aeromonas: An expanding Panorama of species, disease presentations, and unanswered questions. Clin. Infect. Dis. 1998, 27, 332–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, D.C.; Naylor, C.E.; Smedley, J.G., 3rd; Lukoyanova, N.; Robertson, S.; Moss, D.S.; McClane, B.A.; Basak, A.K. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J. Mol. Biol. 2011, 413, 138–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, J.; Crabtree, J.; Roe, B.A.; Tweten, R.K. The primary structure of Clostridium septicum alpha-toxin exhibits similarity with that of Aeromonas hydrophila aerolysin. Infect. Immun. 1995, 63, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Akiba, T.; Abe, Y.; Kitada, S.; Kusaka, Y.; Ito, A.; Ichimatsu, T.; Katayama, H.; Akao, T.; Higuchi, K.; Mizuki, E.; et al. Crystal structure of the parasporin-2 Bacillus thuringiensis toxin that recognizes cancer cells. J. Mol. Biol. 2009, 386, 121–133. [Google Scholar] [CrossRef]
- Shogomori, H.; Kobayashi, T. Lysenin: A sphingomyelin specific pore-forming toxin. Biochim. Biophys. Acta 2008, 1780, 612–618. [Google Scholar] [CrossRef]
- Degiacomi, M.T.; Iacovache, I.; Pernot, L.; Chami, M.; Kudryashev, M.; Stahlberg, H.; van der Goot, F.G.; Dal Peraro, M. Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism. Nat. Chem. Biol. 2013, 9, 623–629. [Google Scholar] [CrossRef]
- Bokori-Brown, M.; Martin, T.G.; Naylor, C.E.; Basak, A.K.; Titball, R.W.; Savva, C.G. Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein. Nat. Commun. 2016, 7, 11293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, A.R.; Gibert, M.; Popoff, M.; Moss, D.S.; Titball, R.W.; Basak, A.K. Clostridium perfringens epsilon-toxin shows structural similarity to the pore-forming toxin aerolysin. Nat. Struct. Mol. Biol. 2004, 11, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.G.; Clark, A.R.; Naylor, C.E.; Popoff, M.R.; Moss, D.S.; Basak, A.K.; Titball, R.W.; Bokori-Brown, M. The pore structure of Clostridium perfringens epsilon toxin. Nat. Commun. 2019, 10, 2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirauqui, N.; Abriata, L.A.; van der Goot, F.G.; Dal Peraro, M. Structural, physicochemical and dynamic features conserved within the aerolysin pore-forming toxin family. Sci. Rep. 2017, 7, 13932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokori-Brown, M.; Kokkinidou, M.C.; Savva, C.G.; Fernandes da Costa, S.; Naylor, C.E.; Cole, A.R.; Moss, D.S.; Basak, A.K.; Titball, R.W. Clostridium perfringens epsilon toxin H149A mutant as a platform for receptor binding studies. Protein Sci. 2013, 22, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyston, P.C.; Payne, D.W.; Havard, H.L.; Williamson, E.D.; Titball, R.W. Production of a non-toxic site-directed mutant of Clostridium perfringens epsilon-toxin which induces protective immunity in mice. Microbiology 1998, 144 Pt 2, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, R.J.; McLeish, T.C. Coupling of global and local vibrational modes in dynamic allostery of proteins. Biophys. J. 2006, 91, 2055–2062. [Google Scholar] [CrossRef] [Green Version]
- McLeish, T.C.; Cann, M.J.; Rodgers, T.L. Dynamic Transmission of Protein Allostery without Structural Change: Spatial Pathways or Global Modes? Biophys. J. 2015, 109, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumah, K.R.; Eleso, O.E.; Fischetti, V.A. Human blood exposure to Clostridium perfringens epsilon toxin may shed light on erythrocyte fragility during active multiple sclerosis. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, V.; Meleard, P.; Pott, T.; Bivas, I. Alamethicin influence on the membrane bending elasticity. Eur. Biophys. J. 2006, 35, 281–286. [Google Scholar] [CrossRef]
- Hale, J.P.; Winlove, C.P.; Petrov, P.G. Effect of hydroperoxides on red blood cell membrane mechanical properties. Biophys. J. 2011, 101, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsitrin, Y.; Morton, C.J.; el-Bez, C.; Paumard, P.; Velluz, M.C.; Adrian, M.; Dubochet, J.; Parker, M.W.; Lanzavecchia, S.; van der Goot, F.G. Conversion of a transmembrane to a water-soluble protein complex by a single point mutation. Nat. Struct. Biol. 2002, 9, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Bokori-Brown, M.; Hall, C.A.; Vance, C.; Fernandes da Costa, S.P.; Savva, C.G.; Naylor, C.E.; Cole, A.R.; Basak, A.K.; Moss, D.S.; Titball, R.W. Clostridium perfringens epsilon toxin mutant Y30A-Y196A as a recombinant vaccine candidate against enterotoxemia. Vaccine 2014, 32, 2682–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Schwoch, G.; Passow, H. Preparation and properties of human erythrocyte ghosts. Mol. Cell. Biochem. 1973, 2, 197–218. [Google Scholar] [CrossRef]
- Bokori-Brown, M.; Petrov, P.G.; Khafaji, M.A.; Mughal, M.K.; Naylor, C.E.; Shore, A.C.; Gooding, K.M.; Casanova, F.; Mitchell, T.J.; Titball, R.W.; et al. Red Blood Cell Susceptibility to Pneumolysin: Correlation with Membrane Biochemical and Physical Properties. J. Biol. Chem. 2016, 291, 10210–10227. [Google Scholar] [CrossRef] [Green Version]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, S.; McGill, B.; Morcrette, H.; Winlove, C.P.; Chimerel, C.; Petrov, P.G.; Bokori-Brown, M. Interaction of Clostridium perfringens Epsilon Toxin with the Plasma Membrane: The Role of Amino Acids Y42, Y43 and H162. Toxins 2022, 14, 757. https://doi.org/10.3390/toxins14110757
Marshall S, McGill B, Morcrette H, Winlove CP, Chimerel C, Petrov PG, Bokori-Brown M. Interaction of Clostridium perfringens Epsilon Toxin with the Plasma Membrane: The Role of Amino Acids Y42, Y43 and H162. Toxins. 2022; 14(11):757. https://doi.org/10.3390/toxins14110757
Chicago/Turabian StyleMarshall, Skye, Beth McGill, Helen Morcrette, C. Peter Winlove, Catalin Chimerel, Peter G. Petrov, and Monika Bokori-Brown. 2022. "Interaction of Clostridium perfringens Epsilon Toxin with the Plasma Membrane: The Role of Amino Acids Y42, Y43 and H162" Toxins 14, no. 11: 757. https://doi.org/10.3390/toxins14110757




