Effects of Compound Mycotoxin Detoxifier on Alleviating Aflatoxin B1-Induced Inflammatory Responses in Intestine, Liver and Kidney of Broilers
Abstract
:1. Introduction
2. Results
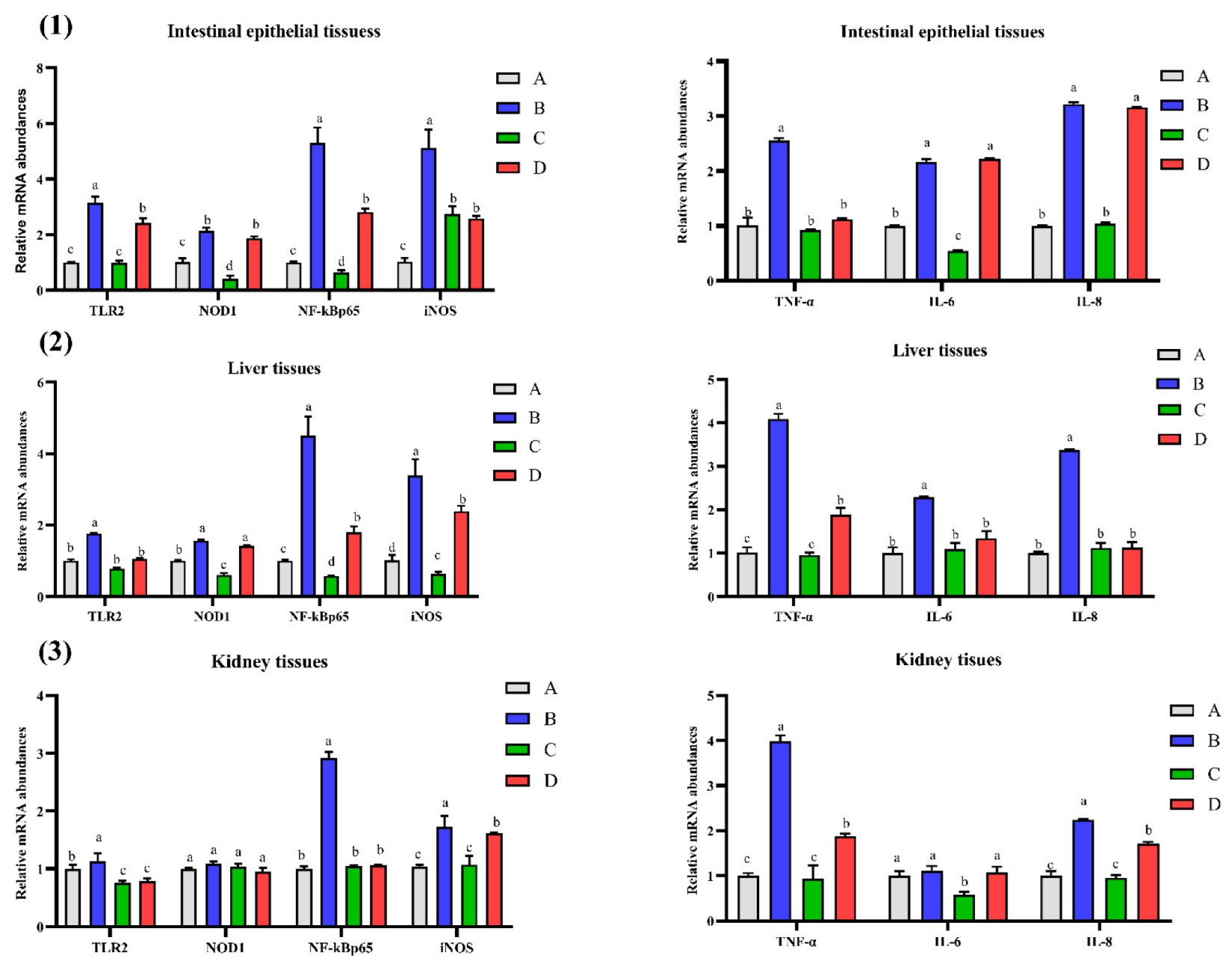
2.1. Effect of CMD on mRNA Abundances of Some Genes in Intestinal, Liver and Kidney Tissues of Broilers
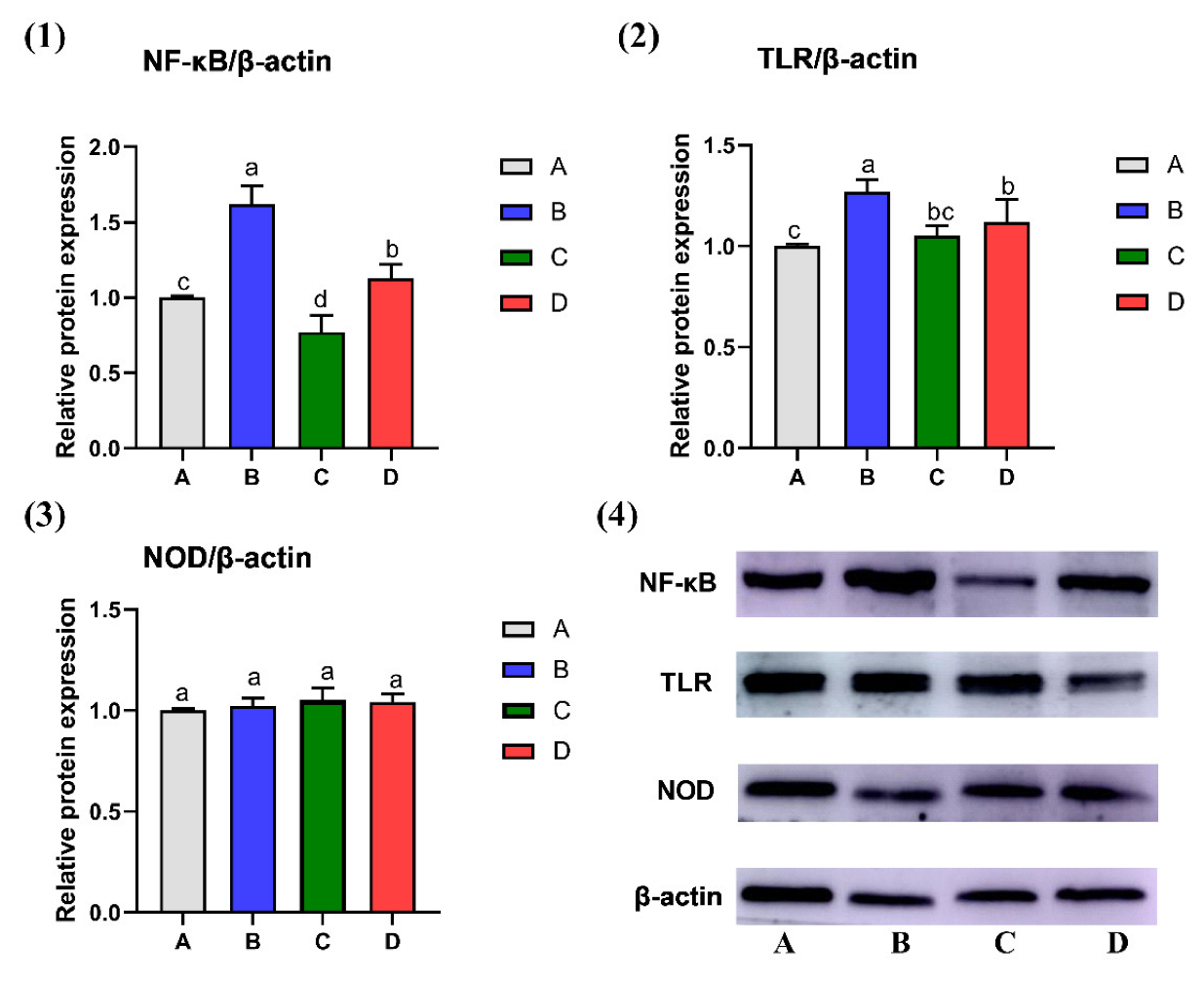
2.2. Effect of CMD on Expression Levels of NF-κB, TLR and NOD in Liver Tissue by WB Analysis
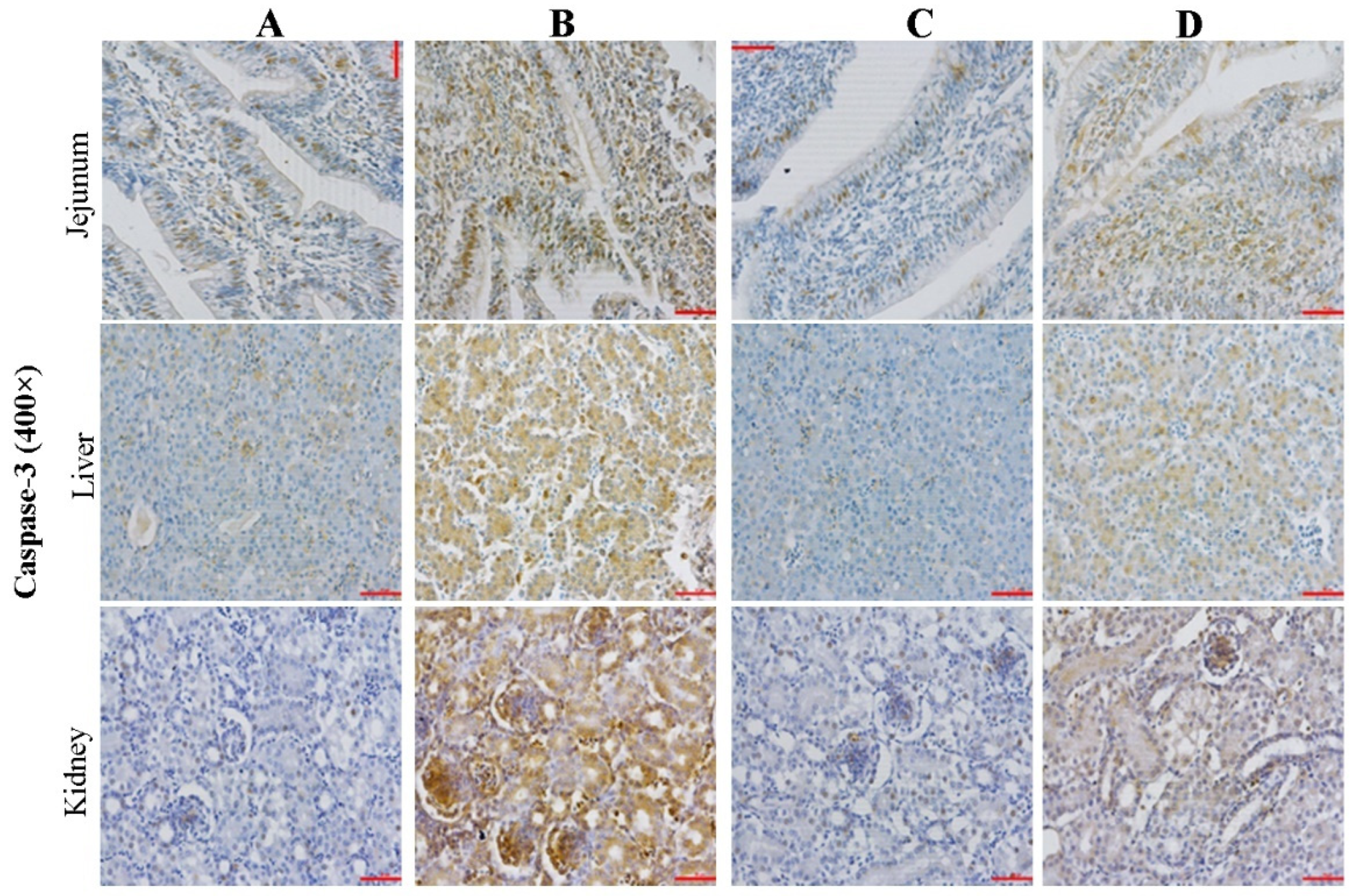
2.3. Effect of CMD on Expression Levels of Caspase-3 in Intestinal, Liver and Kidney Tissues by IHC Analysis
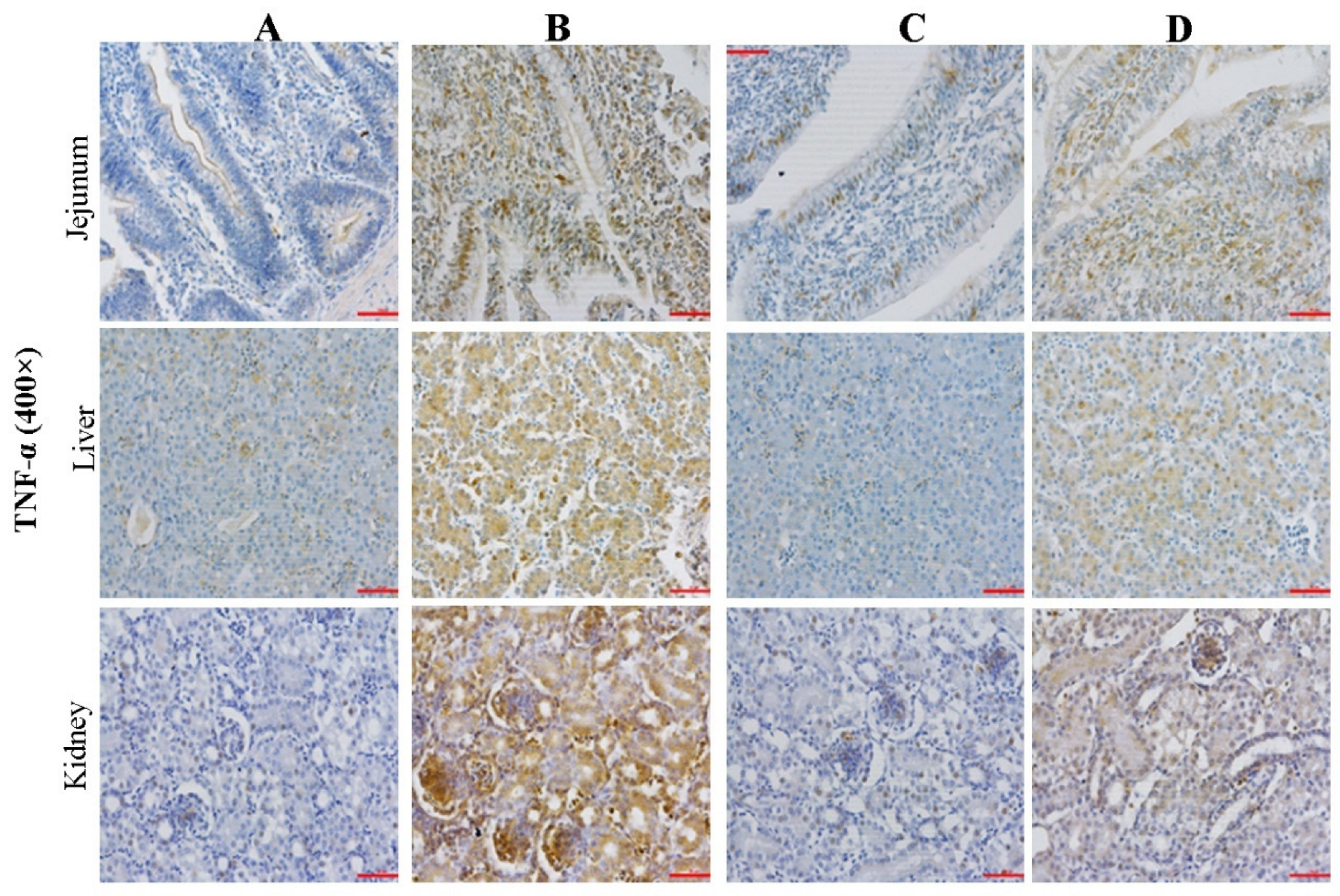
2.4. Effect of CMD on Expression Levels of TNF-α in Intestinal, Liver and Kidney Tissues by IHC Analysis
2.5. Effect of CMD on Expression Levels of NF-κB in Intestinal, Liver and Kidney Tissues by IHC Analysis
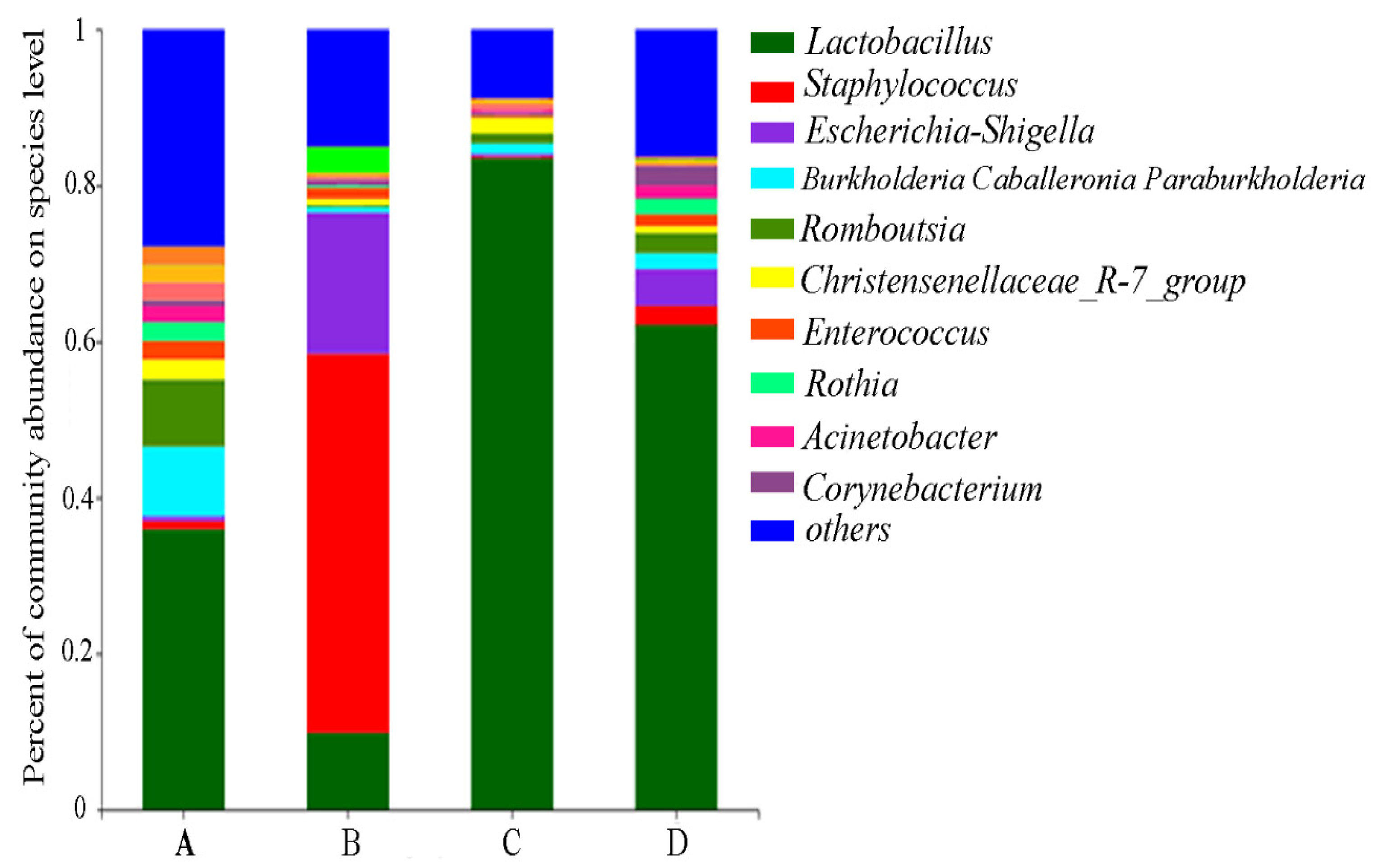
2.6. Gut Microbial Community Influenced by CMD and AFB1
2.7. Effect of CMD on Expression Levels of TNF-α in Intestinal, Liver and Kidney Tissues by IHC Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Compound Mycotoxin Detoxifier (CMD) Preparation
5.2. Moldy Corn Collection and AFB1 Determination
5.3. Animals and Managements
- Group A: Basal diet (4.31 μg/kg AFB1)
- Group B: Basal diet with moldy corn meal (40 μg/kg AFB1)
- Group C: Group A plus 1.5 g/kg CMD
- Group D: Group B plus 1.5 g/kg CMD
5.4. Tissue Collection
5.5. qRT-PCR Analysis
5.6. Immunohistochemical (IHC) Staining
5.7. Western Blotting (WB) Analysis
5.8. Gut microbial Community Influenced by CMD and AFB1
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ting, W.; Chang, C.H.; Szonyi, B.; Gizachew, D. Growth and aflatoxin B1, B2, G1, and G2 production by Aspergillus flavus and Aspergillus parasiticus on ground flax seeds (Linum usitatissimum). J. Food Prot. 2020, 83, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Shi, W.; Lv, P.; Meng, W.; Mao, G.; Gong, C.; Chen, Y.; Wei, Y.; He, X.; Zhao, J.; et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, H.; Hu, C.; Tron, T.; Lin, J.; Wang, J.; Sun, B. Molecular docking studies and in vitro degradation of four aflatoxins (AFB1, AFB2, AFG1, and AFG2) by a recombinant laccase from Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Ates, M.B.; Ortatatli, M. The effects of Nigella sativa seeds and thymoquinone on aflatoxin phase-2 detoxification through glutathione and glutathione-S-transferase alpha-3, and the relationship between aflatoxin B1-DNA adducts in broilers. Toxicon 2021, 193, 86–92. [Google Scholar] [CrossRef]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef]
- Fan, T.; Xie, Y.; Ma, W. Research progress on the protection and detoxification of phytochemicals against aflatoxin B1-induced liver toxicity. Toxicon 2021, 195, 58–68. [Google Scholar] [CrossRef]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin b1 contamination was minimized by insect peptide copa3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021, 268, 115713. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, J.I.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Jesus, S.G.; Jianbo, X.; Sajad, A.; Yun, S.H.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. 2022, 1–22. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Møller, C.; Freire, L.; Rosim, R.E.; Margalho, L.P.; Balthazar, C.F.; Franco, L.T.; Sant’Ana, A.S.; Corassin, C.H.; Rattray, F.P.; de Oliveira, C. Effect of Lactic acid bacteria strains on the growth and aflatoxin production potential of Aspergillus parasiticus, and their ability to bind aflatoxin B1, ochratoxin A, and zearalenone in vitro. Front. Microbiol. 2021, 12, 655386. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol relieved acute liver damage in ducks (anas platyrhynchos) induced by AFB1 via modulation of apoptosis and Nrf2 signaling pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Azeem, N.; Nawaz, M.; Anjum, A.A.; Saeed, S.; Sana, S.; Mustafa, A.; Yousuf, M.R. Activity and anti-aflatoxigenic effect of indigenously characterized probiotic lactobacilli against Aspergillus flavus—A common poultry feed contaminant. Animals 2019, 9, 166. [Google Scholar] [CrossRef]
- Chang, J.; Wang, T.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F.; Gao, T. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020, 194, 110420. [Google Scholar] [CrossRef]
- Phillips, T.D.; Wang, M.; Elmore, S.E.; Hearon, S.; Wang, J.S. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner. 2019, 67, 99–110. [Google Scholar] [CrossRef]
- Kihal, A.; Rodriguez-Prado, M.; Godoy, C.; Cristofol, C.; Calsamiglia, S. In vitro assessment of the capacity of certain mycotoxin binders to adsorb some amino acids and water-soluble vitamins. J. Dairy Sci. 2020, 103, 3125–3132. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Kong, Q.; Ma, Y.; Liu, Y. Detoxification of aflatoxin B1 by Zygosaccharomyces rouxii with solid state fermentation in peanut meal. Toxins 2017, 9, 42. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Efficacy of mycotoxin detoxifiers on health and growth of newly-weaned pigs under chronic dietary challenge of deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef]
- Nasrin, R.; Ali, K.; Kamran, T.; Hassan, S.A.G.M. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin B1. Poult. Sci. 2020, 99, 5896–5906. [Google Scholar] [CrossRef]
- Guo, H.W.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Li, S.; Zhu, Q.; Yang, M.; Hu, X. Detoxification of aflatoxin B1 in broiler chickens by a triple-action feed additive. Food Addit. Contam. A 2021, 38, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.W.; Chang, J.; Wang, P.; Yin, Q.Q.; Liu, C.Q.; Xu, X.X.; Dang, X.W.; Hu, X.F.; Wang, Q.L. Effects of compound probiotics and aflatoxin-degradation enzyme on alleviating aflatoxin-induced cytotoxicity in chicken embryo primary intestinal epithelium, liver and kidney cells. AMB Express 2021, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Xi, Y.; Wang, S.T.; Zheng, L.Y.; Qi, Y.; Guo, S.S.; Ding, B.Y. Effects of Chinese gallnut tannic acid on growth performance, blood parameters, antioxidative status, intestinal histomorphology, and cecal microbial shedding in broilers challenged with aflatoxin B1. J. Anim. Sci. 2022, 100, skac099. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Ishfaq, M.; Wang, J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune responses and Salmonella Pullorum infection resistance of broilers challenged with aflatoxin B1. Poult. Sci. 2022, 101, 101651. [Google Scholar] [CrossRef]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo evaluation of AFB1 and OTA-toxicity through immunofluorescence and flow cytometry techniques: A systematic review. Food Chem. Toxicol. 2022, 16, 112798. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Song, A.; Gao, T.; Dang, X.; Lu, F. Effect of compound probiotics and mycotoxin degradation enzymes on alleviating cytotoxicity of swine jejunal epithelial cells induced by aflatoxin B₁ and zearalenone. Toxins 2019, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Heba, E.M.; Ahmed, A.A.N.; Abdellatif, N.A.; Anani, M.; Fareed, S.A.; El-Shafei, D.A.; Alaa El-Din, E.A. Amelioration of pulmonary aflatoxicosis by green tea extract: An in vivo study. Toxicon 2021, 189, 48–55. [Google Scholar] [CrossRef]
- Jang, B.C.; Paik, J.H.; Kim, S.P.; Bae, J.H.; Mun, K.C.; Song, D.K.; Cho, C.H.; Shin, D.H.; Kwon, T.K.; Park, J.W.; et al. Catalase induces the expression of inducible nitric oxide synthase through activation of NF-kappaB and PI3K signaling pathway in Raw 264.7 cells. Biochem. Pharmacol. 2004, 68, 2167–2176. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, X.; Chen, J.; Zheng, G.; Xie, C.; Wu, H.; Miao, Z.; Lin, Y.; Wang, X.; Gao, W.; et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis. 2021, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.; Lovering, R.C.; Alnemri, E.S.; Bertin, J.; Boss, J.M.; Davis, B.K.; Flavell, R.A.; Girardin, S.E.; Godzik, A.; Harton, J.A.; et al. The NLR gene family: A standard nomenclature. Immunity 2008, 28, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological injuries, ultrastructural changes, and depressed TLR expression in the small intestine of broiler chickens with aflatoxin B₁. Toxins 2018, 10, 131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Liu, M.; Jiang, K.; Wang, M.; Wang, L. Comparative transcriptome analysis reveals the different roles between hepatopancreas and intestine of Litopenaeus vannamei in immune response to aflatoxin B1 (AFB1) challenge. Comp. Biochem. Physiol. C 2019, 222, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Malvandi, A.M.; Mehrzad, J.; Saleh-Moghaddam, M. Biologically relevant doses of mixed aflatoxins B and G up-regulate MyD88, TLR2, TLR4 and CD14 transcripts in human PBMCs. Immunopharmacol. Immunotoxicol. 2013, 35, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ge, J.; Gao, H.; Pan, Y.; Hao, Y.; Li, J. Melatonin attenuates AFB1-induced cardiotoxicity via the NLRP3 signaling pathway. J. Int. Med. Res. 2020, 48, 2656. [Google Scholar] [CrossRef]
- Hernández-Ramírez, J.O.; Nava-Ramírez, M.J.; Merino-Guzmán, R.; Téllez-Isaías, G.; Vázquez-Durán, A.; Méndez-Albores, A. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. 2020, 36, 31–39. [Google Scholar] [CrossRef]
- Moneim, A.; Fahmy, M.F.; Metwally, M.M.; Hassanin, O.; Mowafy, R.E. Ameliorative effects of cholestyramine and oxihumate on aflatoxicosis in broiler chickens. Pak. Vet. J. 2021, 41, 51–56. [Google Scholar]
- Magnoli, A.P.; Rodriguez, M.C.; González Pereyra, M.L.; Poloni, V.L.; Peralta, M.F.; Nilson, A.J.; Miazzo, R.D.; Bagnis, G.; Chiacchiera, S.M.; Cavaglieri, L.R. Use of yeast (Pichia kudriavzevii) as a novel feed additive to ameliorate the effects of aflatoxin B1 on broiler chicken performance. Mycotoxin Res. 2017, 33, 273–283. [Google Scholar] [CrossRef]
- Perali, C.; Magnoli, A.P.; Aronovich, M.; Rosa, C.; Cavaglieri, L.R. Lithothamnium calcareum (Pallas) Areschoug seaweed adsorbs aflatoxin B1 in vitro and improves broiler chicken’s performance. Mycotoxin Res. 2020, 36, 371–379. [Google Scholar] [CrossRef]
- Zuo, R.Y.; Chang, J.; Yin, Q.Q.; Wang, P.; Yang, Y.R.; Wang, X.; Wang, G.Q.; Zheng, Q.H. Effect of the combined probiotics with aflatoxin B₁-degrading enzyme on aflatoxin detoxification, broiler production performance and hepatic enzyme gene expression. Food Chem. Toxicol. 2013, 59, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, B.J.; Liu, K.H.; Hunter, C.S. Gut resident Lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 2020, 31, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.K.; Ashraf, K.; Gul, S.T.; Naseem, M.N.; Khan, A. Toxicopathological effects of feeding aflatoxins b1 in broilers and its ameliosration with indigenous mycotoxin binder. Ecotox. Environ. Safe 2019, 187, 109712. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Cukrowska, B.; Smulikowska, S.; Cielecka-Kuszyk, J. The effect of probiotic supplementation on performance and the histopathological changes in liver and kidneys in broiler chickens fed diets with aflatoxin B₁. Toxins 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Finamore, A.; Roselli, M.; Imbinto, A.; Seeboth, J.; Oswald, I.P.; Mengheri, E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS ONE 2014, 9, e94891. [Google Scholar] [CrossRef]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and heat-killed probiotic Lactobacillus casein Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef]
- Badia, R.; Lizardo, R.; Martinez, P.; Badiola, I.; Brufau, J. The influence of dietary locust bean gum and live yeast on some digestive immunological parameters of piglets experimentally challenged with Escherichia coli. J. Anim. Sci. 2012, 90, 260–262. [Google Scholar] [CrossRef]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Bierla, K.; Jiménez-Lamana, J.; Kot, A.M.; Alcántara-Durán, J.; Piwowarek, K.; Błażejak, S.; Szpunar, J. Metabolic response of the yeast candida utilis during enrichment in selenium. Int. J. Mol. Sci. 2020, 21, 5287. [Google Scholar] [CrossRef] [PubMed]
- Hamza, Z.; El-Hashash, M.; Aly, S.; Hathout, A.; Soto, E.; Sabry, B.; Ostroff, G. Preparation and characterization of yeast cell wall beta-glucan encapsulated humic acid nanoparticles as an enhanced aflatoxin B1 binder. Carbohydr. Polym. 2019, 203, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, R.; Liu, Y.; Ma, L.; Zha, J.; Qiao, X.; Chai, T.; Wu, B. Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrob. 2020, 12, 1385–1397. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, J.; Zhang, H.J.; Wu, S.G.; Hui, Q.R.; Yang, C.B.; Fang, R.J.; Qi, G.H. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Front. Physiol. 2018, 9, 1968. [Google Scholar] [CrossRef]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host-and microbiota-derived extracellular vesicles, immune function, and disease development. Int. J. Mol. Sci. 2019, 21, 107. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Zaffaroni, L.; Peri, F. Recent advances on Toll-like receptor 4 modulation: New therapeutic perspectives. Future Med. Chem. 2018, 10, 461–476. [Google Scholar] [CrossRef]
- Moses, A.K.; Ghazi, T.; Naidoo, D.B.; Chuturgoon, A. DNA methylation of MEKKK1: A strategy to reactivate the NF-κB pathway and reverse HIV latency. AIDS 2021, 35, 2221–2224. [Google Scholar] [CrossRef]
- Hou, L.; Zhou, X.; Gan, F.; Liu, Z.; Zhou, Y.; Qian, G.; Huang, K. Combination of selenomethionine and N-Acetylcysteine alleviates the joint toxicities of aflatoxin B1 and ochratoxin a by ERK MAPK signal pathway in porcine alveolar macrophages. J. Agric. Food Chem. 2018, 66, 5913–5923. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
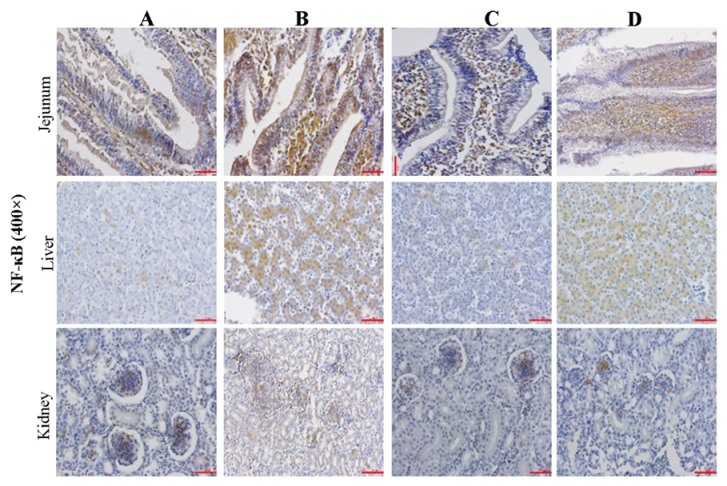
| Groups | A | B | C | D |
|---|---|---|---|---|
| PRC (%) | ||||
| Intestine | 27.73 ± 1.01 A,b | 50.57 ± 2.06 B,a | 19.35 ± 2.65 A,c | 22.14 ± 2.1 A,bc |
| Liver | 11.71 ± 1.52 B,b | 49.25 ± 2.4 B,a | 10.44 ± 1.95 B,b | 13.72 ± 1.24 B,b |
| Kidney | 3.51 ± 2.48 C,c | 69.87 ± 1.33 A,a | 25.24 ± 3.92 A,b | 26.12 ± 4.05 A,b |
| COD | ||||
| Intestine | 17.71 ± 1.4 A,b | 20.37 ± 1.04 C,a | 11.87 ± 2.27 A,c | 7.14 ± 0.62 C,d |
| Liver | 10.68 ± 0.37 B,c | 143.2 ± 14.18 A,a | 9.94 ± 0.7 A,c | 43.86 ± 3.92 A,b |
| Kidney | 7.60 ± 0.66 C,c | 85.04 ± 5.67 B,a | 5.27 ± 0.34 B,c | 28.1 ± 4.08 B,b |
| Groups | A | B | C | D |
|---|---|---|---|---|
| PRC (%) | ||||
| Intestine | 0.02 ± 0.01 A,c | 26.19 ± 2.3 B,a | 8.86 ± 3.07 A,b | 7.15 ± 1.71 C,b |
| Liver | 1.36 ± 1.39 A,c | 32.1 ± 3.37 A,a | 0.00 ± 0.00 B,c | 11.53 ± 2.8 B,b |
| Kidney | 0.00 ± 0.00 A,c | 23.73 ± 2.15 B,a | 3.27 ± 2.33 A,b | 19.2 ± 2.93 A,a |
| COD | ||||
| Intestine | 7.60 ± 0.71 C,c | 79.12 ± 4.18 B,a | 7.18 ± 0.21 A,c | 26.48 ± 3.83 B,b |
| Liver | 18.06 ± 1.07 A,d | 124.67 ± 9.33 A,a | 3.07 ± 0.73 B,c | 60.99 ± 4.55 A,b |
| Kidney | 9.45 ± 0.93 B,c | 34.24 ± 2.62 C,a | 4.4 ± 0.28 B,d | 12.01 ± 1.51 C,b |
| Groups | A | B | C | D |
|---|---|---|---|---|
| PRC (%) | ||||
| Intestine | 20.27 ± 3.06 A,b | 83.63 ± 2.84 A,a | 6.47 ± 1.76 A,c | 25.04 ± 2.55 A,b |
| Liver | 1.08 ± 1.52 B,b | 13.52 ± 2.96 B,a | 0.00 ± 0.00 B,b | 7.8 ± 1.82 B,a |
| Kidney | 1.23 ± 1.75 B,b | 13.51 ± 1.85 B,a | 1.33 ± 1.89 B,b | 2.62 ± 1.86 C,b |
| COD | ||||
| Intestine | 15.8 ± 1.21 A,b | 27.12 ± 0.65 B,a | 15.57 ± 0.57 A,b | 15.67 ± 0.33 B,b |
| Liver | 11.7 ± 2.26 B,c | 39.39 ± 5.64 A,a | 13.2 ± 0.81 B,c | 23.25 ± 2.51 A,b |
| Kidney | 11.68 ± 1.84 B,b | 35.55 ± 4.05 A,a | 13.18 ± 1.78 B,b | 7.56 ± 1.24 C,c |
| Groups | A | B | C | D | |
|---|---|---|---|---|---|
| EC 2.7.11.1 | Interleukin-1 receptor-associated kinase (IRAK-1) | 42,321 ± 385 b | 686,817 ± 416 a | 27,456 ± 321 c | 47,551 ± 316 b |
| EC 2.7.10.2 | Janus tyrosine Kinase (JAK) | 212 ± 1.78 b | 57.21 ± 1.32 c | 223.38 ± 12.36 a | 211.87 ± 10.1 a |
| EC2.7.11.25 | Mitogen-activated protein kinase (MAPK) | 25,357 ± 417 a | 30,569 ± 396 a | 14,532 ± 677 b | 27,651 ± 386 a |
| Gene | Accession Number | Primer Sequence (5′–3′) |
|---|---|---|
| β-actin | LO8165 | F: GAGAAATTGTGCGTGACATCA |
| R: CCTGAACCTCTCATTGCCA | ||
| IL-6 | AJ309540 | F: CAAGGTGACGGAGGAGGAC |
| R: TGGCGAGGAGGGATTTCT | ||
| IL-8 | AJ009800 | F: ATGAACGGCAAGCTTGGAGCTG |
| R: TCCAAGCACACCTCTCTTCCATCC | ||
| iNOS | U46504 | F: CAGCTGATTGGGTGTGGAT |
| R: TTTCTTTGGCCTACGGGTC | ||
| NF-κBp65 | NM_205129 | F: GTGTGAAGAAACGGGAACTG |
| R: GGCACGGTTGTCATAGATGG | ||
| TNF-α | NM_204267 | F: GAGCGTTGACTTGGCTGTC |
| R: AAGCAACAACCAGCTATGCAC | ||
| NOD1 | JX465487 | F: AGCACTGTCCATCCTCTGTCC |
| R: TGAGGGTTGGTAAAGGTCTGCT | ||
| TLR2 | NM_001161650 | F: GGGGCTCAGGCAAAATC |
| R: AGCAGGGTTCTCAGGTTCACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Wang, P.; Liu, C.; Zhou, T.; Chang, J.; Yin, Q.; Wang, L.; Jin, S.; Zhu, Q.; Lu, F. Effects of Compound Mycotoxin Detoxifier on Alleviating Aflatoxin B1-Induced Inflammatory Responses in Intestine, Liver and Kidney of Broilers. Toxins 2022, 14, 665. https://doi.org/10.3390/toxins14100665
Guo H, Wang P, Liu C, Zhou T, Chang J, Yin Q, Wang L, Jin S, Zhu Q, Lu F. Effects of Compound Mycotoxin Detoxifier on Alleviating Aflatoxin B1-Induced Inflammatory Responses in Intestine, Liver and Kidney of Broilers. Toxins. 2022; 14(10):665. https://doi.org/10.3390/toxins14100665
Chicago/Turabian StyleGuo, Hongwei, Ping Wang, Chaoqi Liu, Ting Zhou, Juan Chang, Qingqiang Yin, Lijun Wang, Sanjun Jin, Qun Zhu, and Fushan Lu. 2022. "Effects of Compound Mycotoxin Detoxifier on Alleviating Aflatoxin B1-Induced Inflammatory Responses in Intestine, Liver and Kidney of Broilers" Toxins 14, no. 10: 665. https://doi.org/10.3390/toxins14100665





