Serodiagnosis and Bacterial Genome of Helicobacter pylori Infection
Abstract
:1. Introduction
2. Results
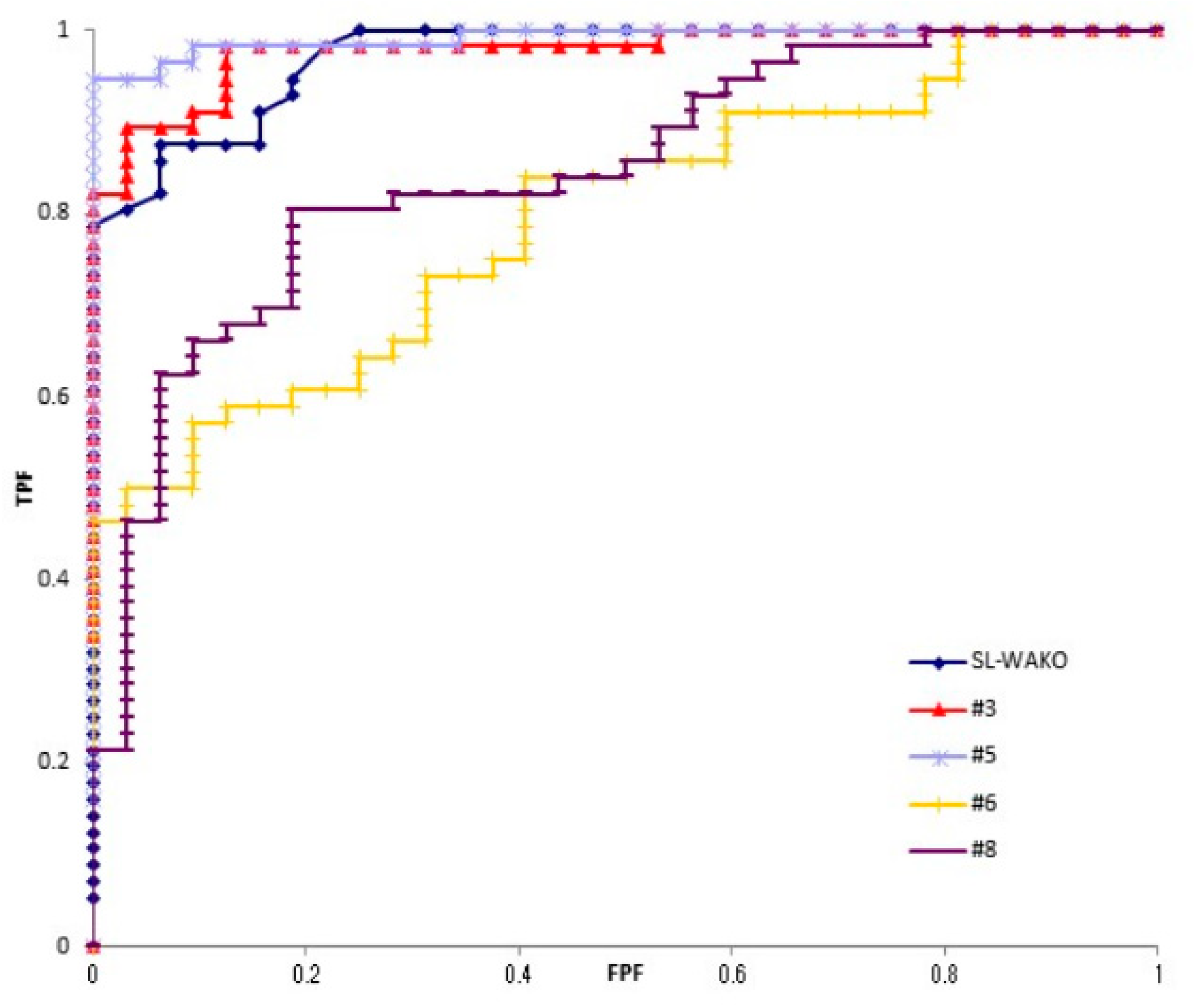
2.1. Antibody Reaction of H. pylori Strains in the Patients
2.2. Characteristics of Four Genome
2.3. Number and Name of Common Genes
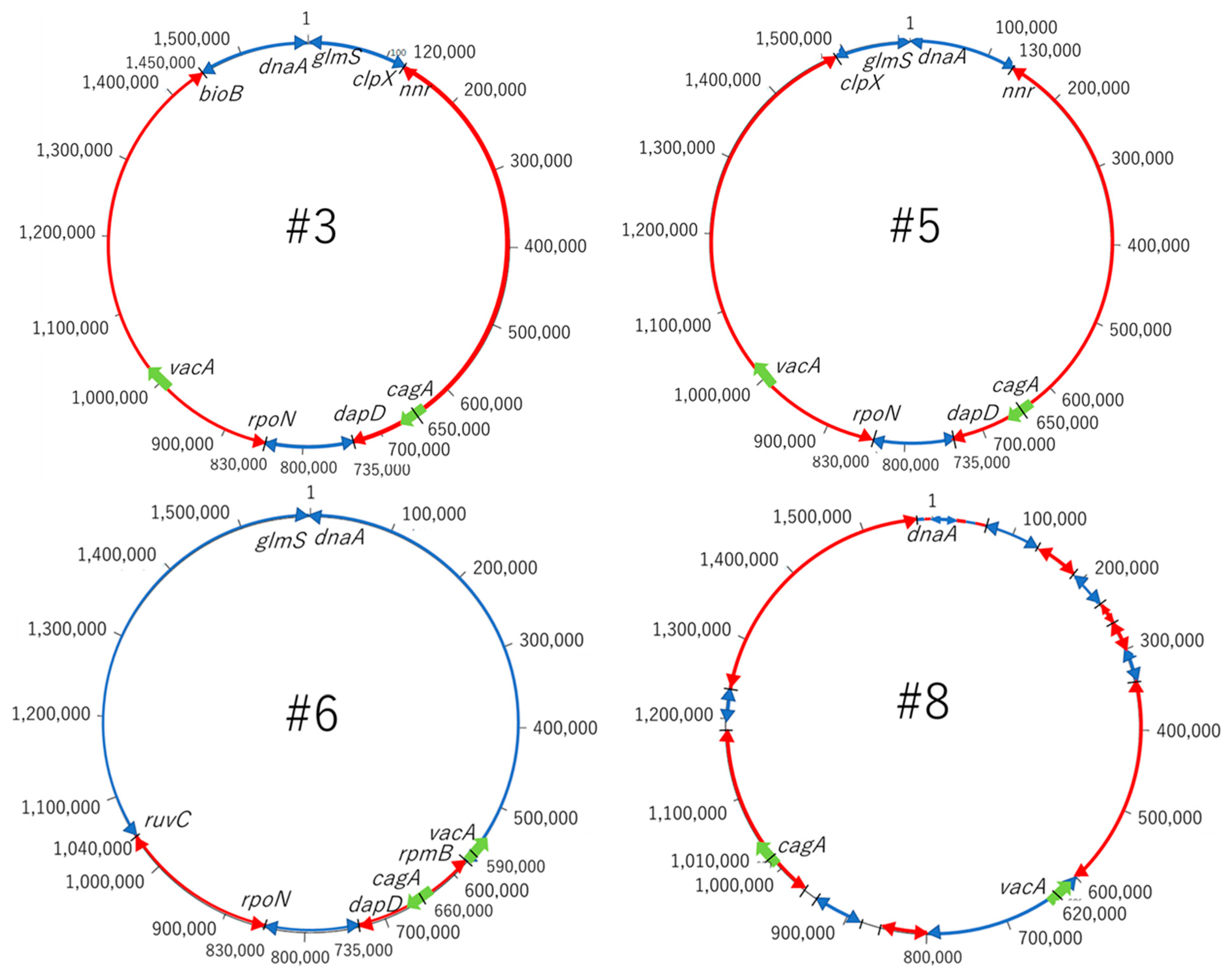
2.4. Genome Ring
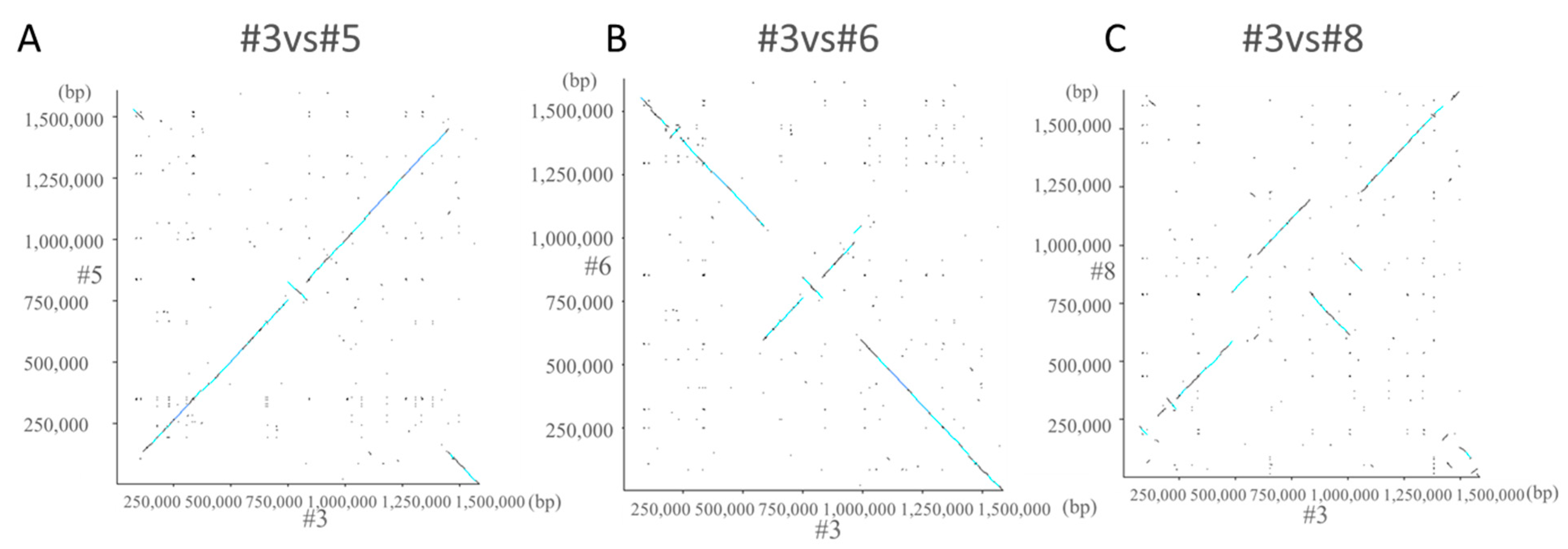
2.5. Dot Plot Analysis
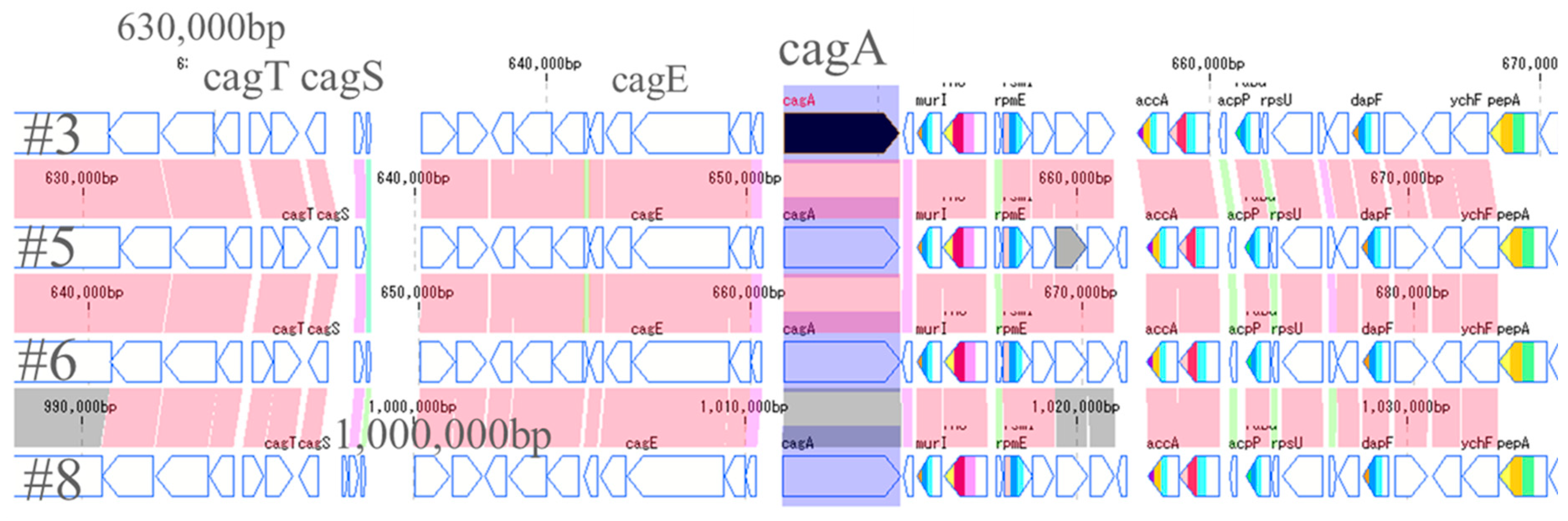
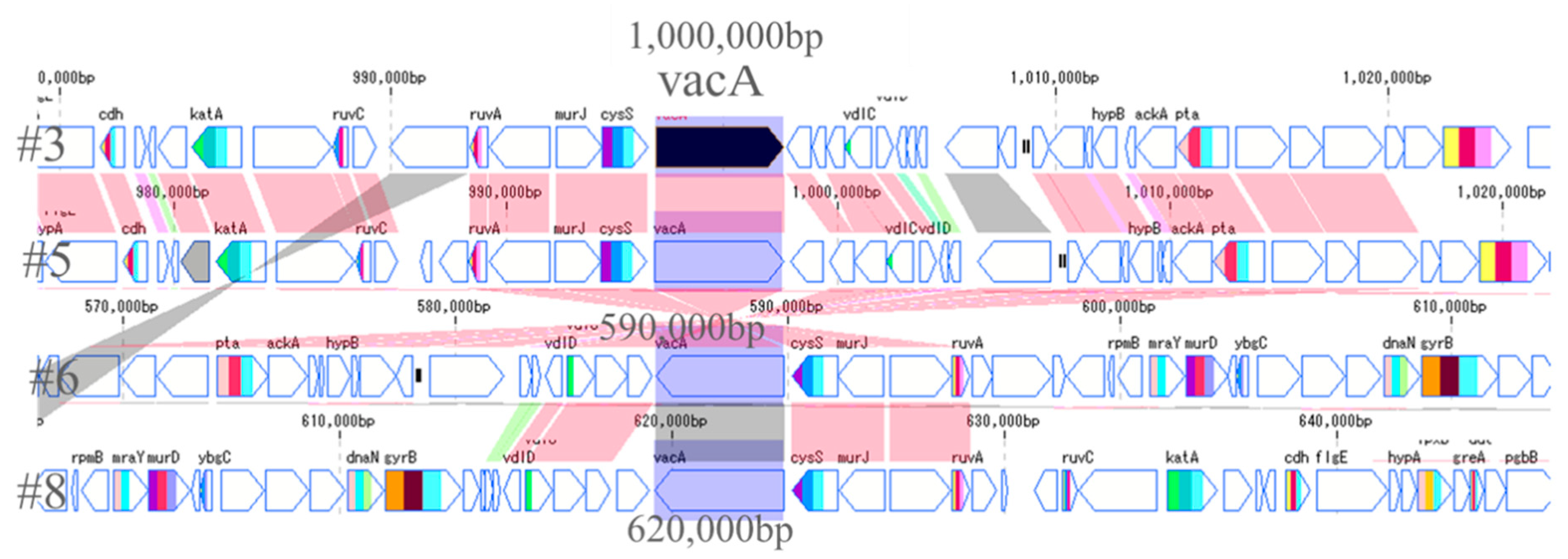
2.6. CagA and VacA Gene
2.7. CagA and VacA Protein Expression Level
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. DNA Extraction
4.3. Whole Genome Sequence
4.4. Genome Analysis
4.5. VacA and CagA Protein Expression
4.6. Western Blot
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Infection with Helicobacter pylori. In Schistosomes, Liver Flukes and Helicobacter pylori; IARC Monographs: Lyon, France, 1994; Volume 61, pp. 177–240. [Google Scholar]
- Blaser, M.J. The bacteria behind ulcers. Sci. Am. 1996, 274, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y. Helicobacter pylori infection is the primary cause of gastric cancer. J. Gastroenterol. 2000, 35 (Suppl. 12), 90–97. [Google Scholar] [PubMed]
- de Martel, C.; Forman, D.; Plummer, M. Gastric cancer: Epidemiology and risk factors. Gastroenterol. Clin. 2013, 42, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y. Incidence and mortality of gastric cancer in China. World J. Gastroenterol. 2006, 12, 17–20. [Google Scholar]
- Wang, Y.K.; Kuo, F.C.; Liu, C.J.; Wu, M.C.; Shih, H.Y.; Wang, S.S.; Wu, J.-Y.; Kuo, C.-H.; Huang, Y.-K.; Wu, D.-C. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 2015, 28, 11221–11235. [Google Scholar] [CrossRef]
- Newell, D.G.; Stacey, A.R. The serology of Campylobacter pylori infections. In Campylobacter pylori and Gastroduodenal Disease; Blackwell Scientific Publications, Ltd.: Oxford, UK, 1989; pp. 74–82. [Google Scholar]
- Perez-Perez, G.I.; Dunn, B.E. Diagnosis of C. pylori infection by serological methods. In Campylobacter pylori in Gastritis and Peptic Ulcer Disease; Igaku-Shoin: New York, NY, USA, 1989; pp. 163–174. [Google Scholar]
- Suzuki, H.; Masaoka, T.; Nomura, S.; Hoshino, Y.; Kurabayashi, K.; Minegishi, Y.; Suzuki, M.; Ishii, H. Current consensus on the diagnosis and treatment of H. pylori-associated gastroduodenal disease. Keio J. Med. 2003, 52, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Chey, W.D.; Murthy, U.; Shaw, S.; Zawadski, A.; Montague, J.; Linscheer, W.; Laine, L. A comparison of three finger stick, whole blood antibody tests for H. pylori Infection: A Unite States multicenter trial. Am. J. Gastroenterol. 1999, 94, 1512–1516. [Google Scholar] [CrossRef]
- Ho, B.; Marshall, B.J. Accurate diagnosis of H. pylori. serologic testing. Gastroenterol. Clin. N. Am. 2000, 29, 853–862. [Google Scholar] [CrossRef]
- Kita, M.; Take, S.; Okada, H.; Matsushita, O.; Yokota, K. A Study to determine the optimum antigens for the serodiagnosis of Helicobacter pylori infection in Japanese patients and the association with IgG subclass and gastric cancer. Jpn. J. Clin. Pathol. 2015, 63, 180–186. (In Japanese) [Google Scholar]
- Harr, R.; Hagblom, P.; Gustafsson, P. Two-dimensional graphic analysis of DNA sequence homologies. Nucleic Acids Res. 1982, 10, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formichella, L.; Romberg, L.; Bolz, C.; Vieth, M.; Geppert, M.; Göttner, G.; Nölting, C.; Walter, D.; Schepp, W.; Schneider, A.; et al. A novel line immunoassay based on recombinant virulence factors enables highly specific and sensitive serologic diagnosis of Helicobacter pylori infection. Clin. Vaccine Immunol. 2013, 20, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Gholi, M.K.; Kalali, B.; Formichella, L.; Göttner, G.; Shamsipour, F.; Zarnani, A.H.; Hosseini, M.; Busch, D.H.; Shirazi, M.H.; Gerhard, M. Helicobacter pylori FliD protein is a highly sensitive and specific marker for serologic diagnosis of H. pylori infection. Int. J. Med. Microbiol. 2013, 303, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.F.; Formichella, L.; Zhang, L.; Zhang, Y.; Ma, J.L.; Li, Z.X.; Liu, C.; Wang, Y.M.; Goettner, G.; Ulm, K.; et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int. J. Cancer 2014, 134, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Marchildon, P.A.; Sugiyama, T.; Fukuda, Y.; Peacock, J.S.; Asaka, M.; Shimoyama, T.; Graham, D.Y. Evaluation of the effects of strain- specific antigen variation on the accuracy of serologic diagnosis of Helicobacter pylori infection. J. Clin. Microbiol. 2003, 41, 1480–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, T.T.; Wheeldon, T.U.; Bengtsson, C.; Phung, D.C.; Sörberg, M.; Granström, M. Enzyme-linked immunosorbent assay for Helicobacter pylori needs adjustment for the population investigated. J. Clin. Microbiol. 2004, 42, 627–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Strain | Total Length (bp) | GC Content (%) | No. of CDSs | No. of rRNA | No. of tRNA |
|---|---|---|---|---|---|
| #3 | 1,585,790 | 38.8 | 1518 | 4 | 36 |
| #5 | 1,608,283 | 38.7 | 1565 | 4 | 36 |
| #6 | 1,630,060 | 38.7 | 1557 | 4 | 36 |
| #8 | 1,668,167 | 38.8 | 1607 | 4 | 36 |
| Gene Name | Mw (kDa) | Function |
|---|---|---|
| Common between strain #3 and #5 | ||
| eptA | 58.8 | Resistance to antibiotics and metal ions |
| ddl | 39.4 | Metabolism of D-alanine, peptidoglycan Involved in biosynthesis |
| ruvC | 17.4 | Elimination of holiday structure |
| alr | 41.8 | Catalyzing the conversion of L-alanine and D-alanine |
| ybeY | 16.1 | Center of gene regulation via sRNA |
| xseB | 7.6 | Single-strand DNA endonuclease |
| Common between strain #6 and #8 | ||
| exbD | 16.6 | Involved in bacterial iron transport |
| metN | 36.6 | Involved in methionine uptake |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichihara, A.; Ojima, H.; Gotoh, K.; Matsushita, O.; Take, S.; Okada, H.; Watanabe, A.; Yokota, K. Serodiagnosis and Bacterial Genome of Helicobacter pylori Infection. Toxins 2021, 13, 467. https://doi.org/10.3390/toxins13070467
Ichihara A, Ojima H, Gotoh K, Matsushita O, Take S, Okada H, Watanabe A, Yokota K. Serodiagnosis and Bacterial Genome of Helicobacter pylori Infection. Toxins. 2021; 13(7):467. https://doi.org/10.3390/toxins13070467
Chicago/Turabian StyleIchihara, Aina, Hinako Ojima, Kazuyoshi Gotoh, Osamu Matsushita, Susumu Take, Hiroyuki Okada, Akari Watanabe, and Kenji Yokota. 2021. "Serodiagnosis and Bacterial Genome of Helicobacter pylori Infection" Toxins 13, no. 7: 467. https://doi.org/10.3390/toxins13070467





