Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells
Abstract
:1. Introduction
2. Results
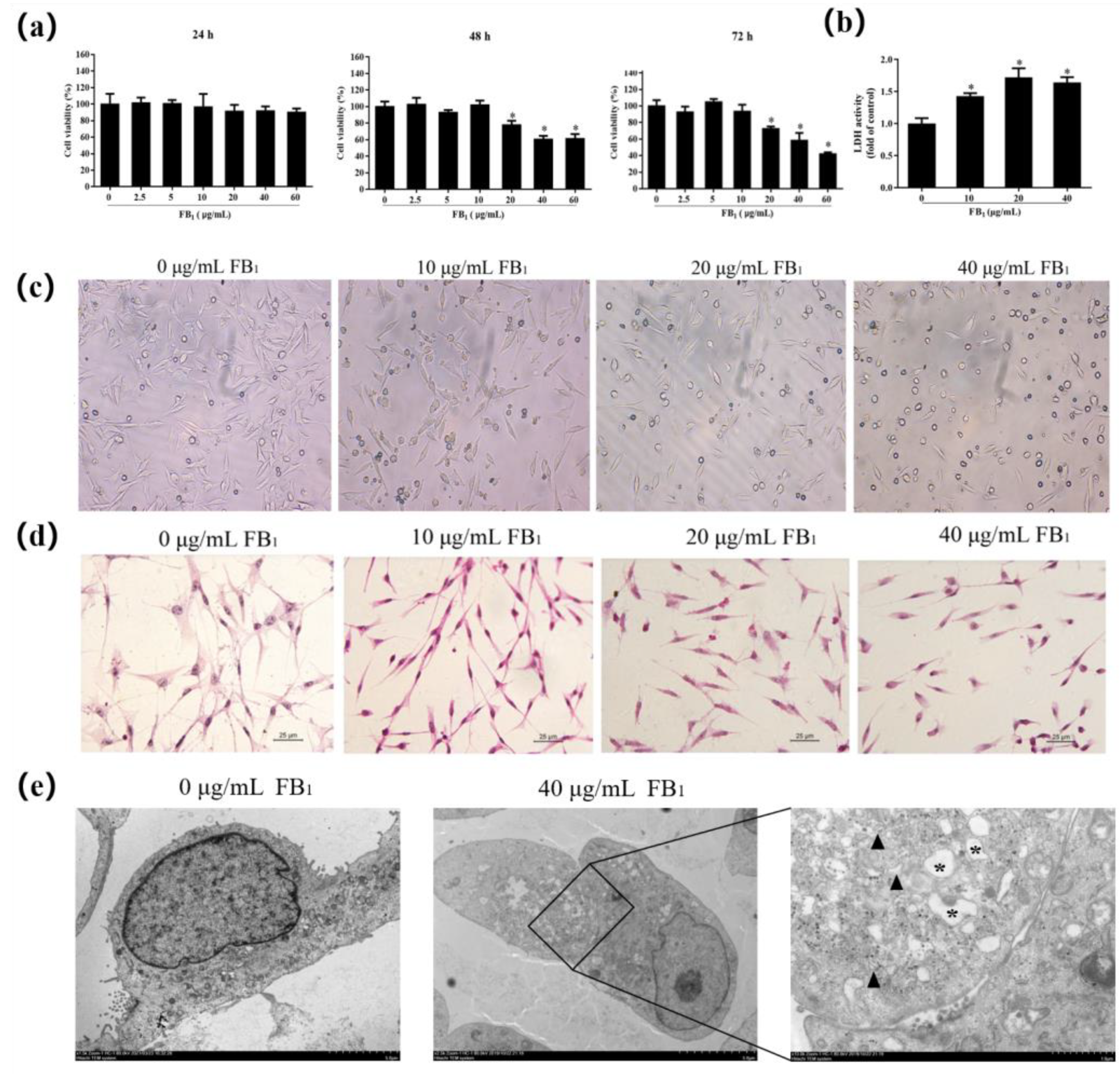
2.1. FB1 Reduces Cell Viability and Causes Cell Damage
2.2. FB1 Inhibits Proliferation of SUVECs by Blocking the Cell Cycle
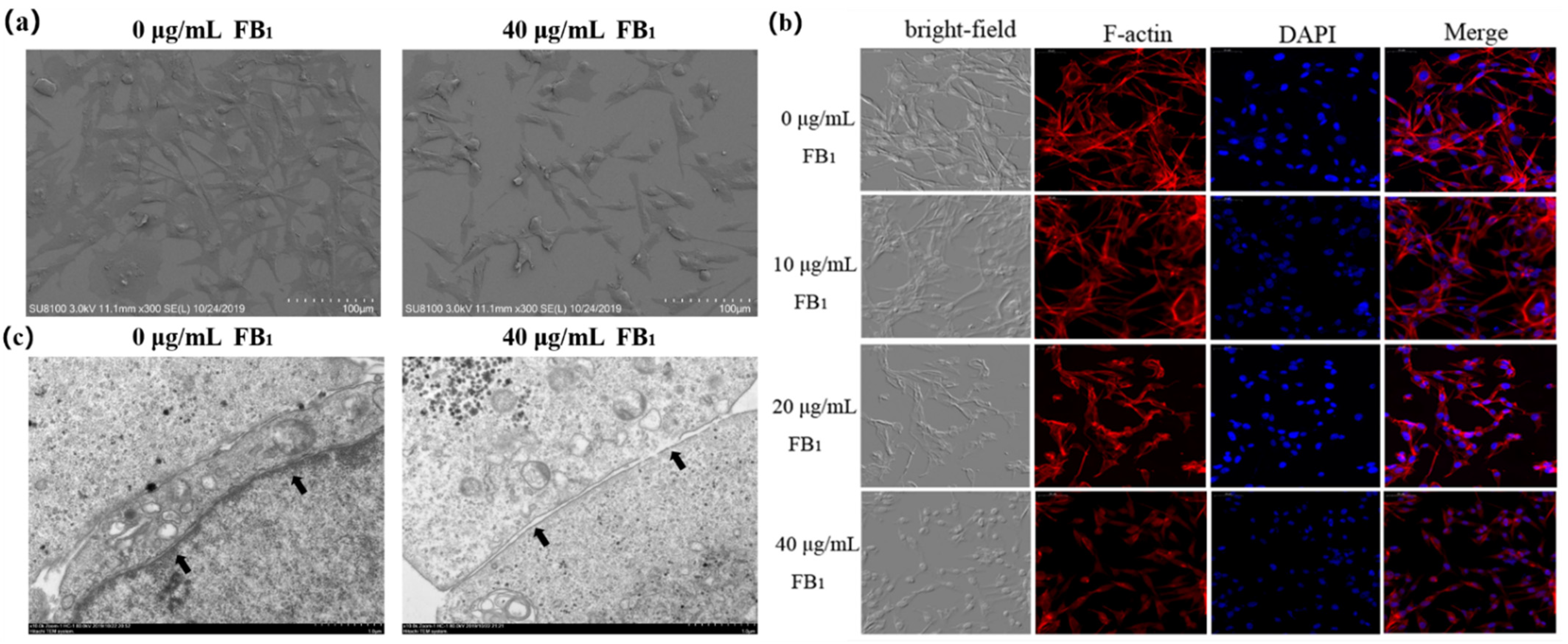
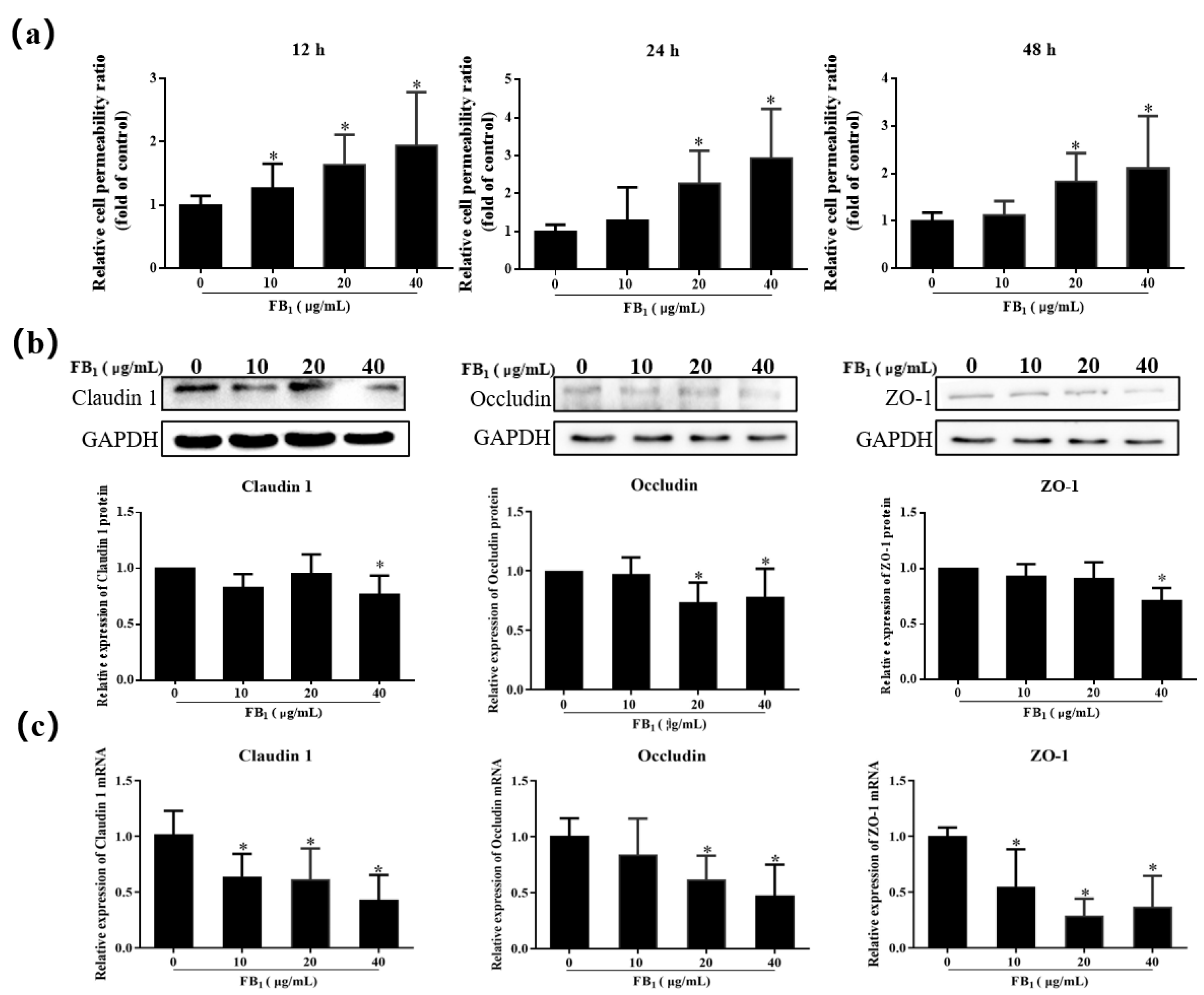
2.3. FB1 Destroys the Tight Junctions of SUVECs
2.4. FB1 Increases the Cell Paracellular Permeability of SUVECs
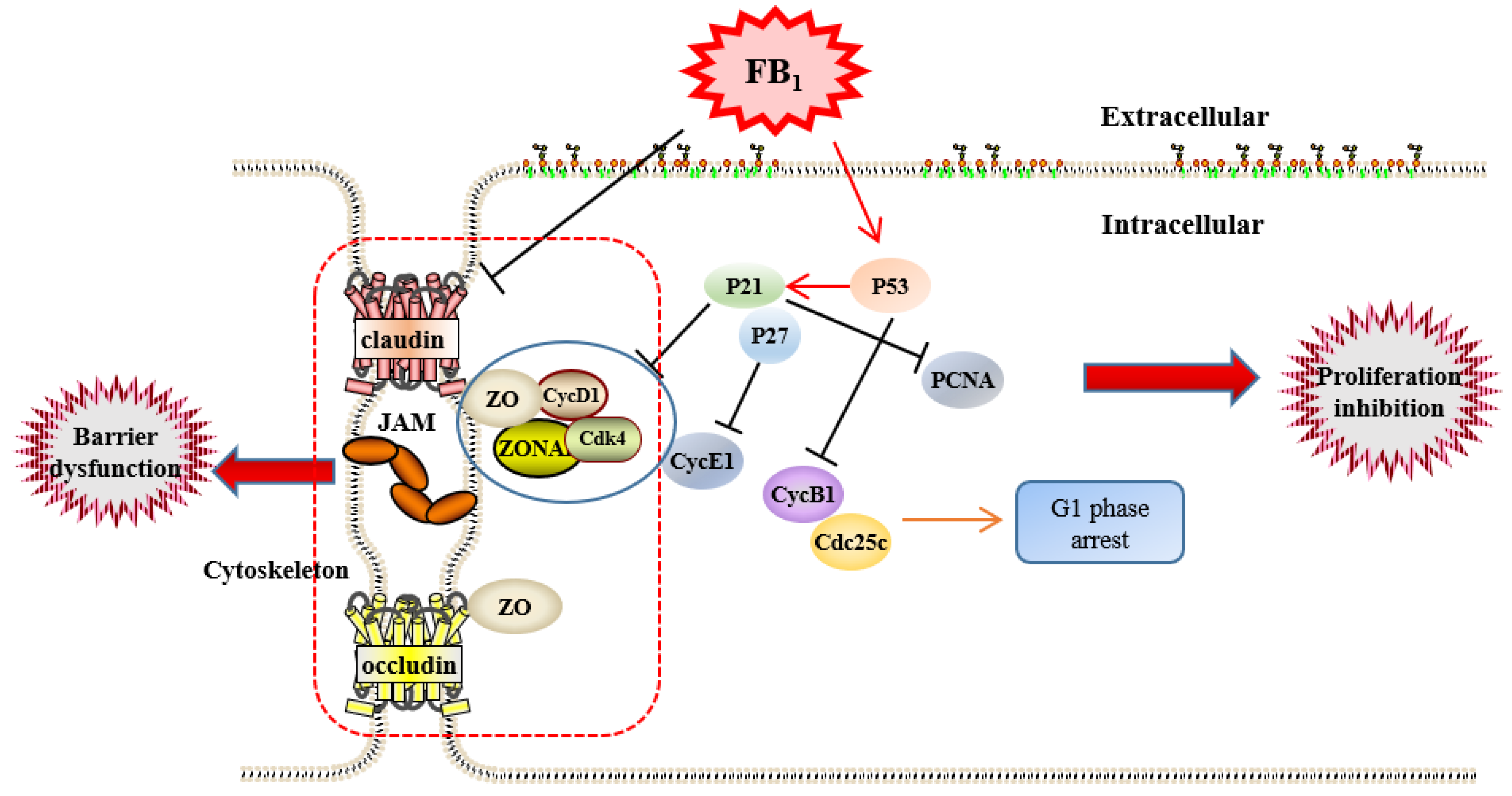
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell and Cell Culture
5.2. Cell Viability Assay
5.3. LDH Cytotoxicity Assay
5.4. HE Staining
5.5. Flow Cytometric Analysis
5.6. qRT-PCR Assay
5.7. Western Blot
5.8. Scanning Electron Microscopy (SEM)
5.9. Transmission Electron Microscopy (TEM)
5.10. Paracellular Permeability Testing
5.11. Phalloidin Staining
5.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, P.M. Fumonisins. Int. J. Food Microbiol. 1993, 18, 257–270. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Li, C.Y.; Yao, N. Fumonisin B1: A Tool for Exploring the Multiple Functions of Sphingolipids in Plants. Front. Plant Sci. 2020, 11, 600458. [Google Scholar] [CrossRef] [PubMed]
- Sheik Abdul, N.; Marnewick, J.L. Fumonisin B (1)—induced mitochondrial toxicity and hepatoprotective potential of rooibos: An update. J. Appl. Toxicol. 2020, 40, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Domijan, A.M. Fumonisin B (1): A neurotoxic mycotoxin. Arh. Za Hig. Rada I Toksikol. 2012, 63, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, S.; Abel, S.; Swanevelder, S.; Gelderblom, W.C. Induction of an altered lipid phenotype by two cancer promoting treatments in rat liver. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 78, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.M.; Abel, S.; Gelderblom, W.C.A. Modulation of key lipid raft constituents in primary rat hepatocytes by fumonisin B (1)—Implications for cancer promotion in the liver. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Abdul, N.S.; Chuturgoon, A.A. Fumonisin B (1) regulates LDL receptor and ABCA1 expression in an LXR dependent mechanism in liver (HepG2) cells. Toxicon Off. J. Int. Soc. Toxinol. 2021, 190, 58–64. [Google Scholar] [CrossRef]
- Harrison, L.R.; Colvin, B.M.; Greene, J.T.; Newman, L.E.; Cole, J.R. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 1990, 2, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef]
- Colvin, B.M.; Cooley, A.J.; Beaver, R.W. Fumonisin toxicosis in swine: Clinical and pathologic findings. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 1993, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: An overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gumprecht, L.A.; Beasley, V.R.; Weigel, R.M.; Parker, H.M.; Tumbleson, M.E.; Bacon, C.W.; Meredith, F.I.; Haschek, W.M. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: Morphological and biochemical alterations. Toxicol. Pathol. 1998, 26, 777–788. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.Y. Cell Cycle Control: A System of Interlinking Oscillators. Methods Mol. Biol. 2016, 1342, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Bakkar, N. Modulating cell cycle: Current applications and prospects for future drug development. Curr. Cancer Drug Targets 2002, 2, 309–336. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, S.; Meng, X.; Song, F.; Huo, W.; Zhang, S.; Chang, J.; Li, J.; Zheng, B.; et al. Coxsackievirus A6 Induces Cell Cycle Arrest in G0/G1 Phase for Viral Production. Front. Cell. Infect. Microbiol. 2018, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.S.; Nelson, W.J. VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 2010, 22, 651–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejana, E. Endothelial cell-cell junctions: Happy together. Nat. Reviews. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Radhakrishna, H.; Al-Awar, O.; Khachikian, Z.; Donaldson, J.G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 1999, 112, 855–866. [Google Scholar] [CrossRef]
- Hohmann, T.; Dehghani, F. The Cytoskeleton-A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Jia, B.; Liu, N.; Yu, D.; Wu, A. Evaluation of the Individual and Combined Toxicity of Fumonisin Mycotoxins in Human Gastric Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 5917. [Google Scholar] [CrossRef]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A. Fumonisin B (1) Epigenetically Regulates PTEN Expression and Modulates DNA Damage Checkpoint Regulation in HepG2 Liver Cells. Toxins 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Gumprecht, L.A.; Smith, G.W.; Constable, P.C.; Haschek, W.M. Species and organ specificity of fumonisin-induced endothelial alterations: Potential role in porcine pulmonary edema. Toxicology 2001, 160, 71–79. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Liu, J.L.; Zhang, J.H.; Zhang, S.C.; Ouyang, Y.; Huang, J.T.; Peng, X.Y.; Zeng, Z.; Hu, Z.Q. Fumonisin B1 Affects the Biophysical Properties, Migration and Cytoskeletal Structure of Human Umbilical Vein Endothelial Cells. Cell Biochem. Biophys. 2020, 78, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Jiang, Y.; Fan, Y.; Ma, Y.; Lei, H.; Su, J. Fumonisin B (1) Induces Oxidative Stress and Breaks Barrier Functions in Pig Iliac Endothelium Cells. Toxins 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef] [PubMed]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of Lactate Dehydrogenase for Cell Viability Testing Using Cell Lines and Primary Cultured Astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Dalton, S. Linking the Cell Cycle to Cell Fate Decisions. Trends Cell Biol. 2015, 25, 592–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 77, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, K.N.; Moldovan, G.L. Forging Ahead through Darkness: PCNA, Still the Principal Conductor at the Replication Fork. Mol. Cell 2017, 65, 380–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.W.; Liu, F. Novel insights into cell cycle regulation of cell fate determination. J. Zhejiang Univ. Sci. B 2019, 20, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kozar, K.; Sicinski, P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle 2005, 4, 388–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 213. [Google Scholar] [CrossRef]
- Giono, L.E.; Resnick-Silverman, L.; Carvajal, L.A.; St Clair, S.; Manfredi, J.J. Mdm2 promotes Cdc25C protein degradation and delays cell cycle progression through the G2/M phase. Oncogene 2017, 36, 6762–6773. [Google Scholar] [CrossRef] [Green Version]
- Ciacci-Zanella, J.R.; Merrill, A.H., Jr.; Wang, E.; Jones, C. Characterization of cell-cycle arrest by fumonisin B1 in CV-1 cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998, 36, 791–804. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef]
- Butler, J.E.; Spear, B.T. Normal intestinal epithelial cell differentiation in the absence of p21 and p27: New insights from old knock-out mice. Cancer Biol. Ther. 2008, 7, 880–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- St Clair, S.; Giono, L.; Varmeh-Ziaie, S.; Resnick-Silverman, L.; Liu, W.J.; Padi, A.; Dastidar, J.; DaCosta, A.; Mattia, M.; Manfredi, J.J. DNA damage-induced downregulation of Cdc25C is mediated by p53 via two independent mechanisms: One involves direct binding to the cdc25C promoter. Mol. Cell 2004, 16, 725–736. [Google Scholar] [CrossRef]
- Haglund, C.M.; Welch, M.D. Pathogens and polymers: Microbe-host interactions illuminate the cytoskeleton. J. Cell Biol. 2011, 195, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.; Izquierdo, M.C.; de Nicolás, R.; Egido, J.; Ortiz, A.; González-Cabrero, J. Gliotoxin inhibits neointimal hyperplasia after vascular injury in rats. J. Vasc. Res. 2009, 46, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Baltierra-Uribe, S.L.; García-Vásquez Mde, J.; Castrejón-Jiménez, N.S.; Estrella-Piñón, M.P.; Luna-Herrera, J.; García-Pérez, B.E. Mycobacteria entry and trafficking into endothelial cells. Can. J. Microbiol. 2014, 60, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, Z.; Hang, X.; Jiang, Y.L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Reviews Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Yi, X.J.; Yu, F.S. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp. Eye Res. 2004, 78, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Yu, L.C.; Wang, J.T.; Wei, S.C.; Ni, Y.H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, G.H. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS ONE 2017, 12, e0189221. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Li, X.; Yuan, Q.; Su, J.; Yang, L.; Ning, L.; Lei, H. Fumonisin B (1) damages the barrier functions of porcine intestinal epithelial cells in vitro. J. Biochem. Mol. Toxicol. 2019, 33, e22397. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Zhao, Y.; Lv, Y.; Hu, Y.; Tan, Y.; Bi, X.; Yu, B.; Kou, J. DT-13 Ameliorates TNF-α-Induced Vascular Endothelial Hyperpermeability via Non-Muscle Myosin IIA and the Src/PI3K/Akt Signaling Pathway. Front. Immunol. 2017, 8, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genes | Primer Sequences | Genebank No |
|---|---|---|
| p21 | F: 5′-ACCCCTTCCCCATACCC-3′ R: 5′-TTCCTAACACCCATGAAACTG-3′ | XM_013977858.2 |
| p27 | F:5′-GTCCCTTTCAGTGAGAACCG ATAC-3′ R: 5′-TTGCTGCCACATAACGGAATCAT-3′ | NM_214316.1 |
| p53 | F: 5′-CTGCTTCCTGAAAACAACC-3′ R: 5′-AAGGGACAAAGGACGACA-3′ | NM_213824.3 |
| PCNA | F: 5′-GTGATTCCACCACCATGTTC-3′ R: 5′-TGAGACGACTCCATGCTCTG-3′ | NM_001291925.1 |
| Cyclin B1 | F: 5′-AACTGCTCTTGGAGACATCGGT-3′ R: 5′-TGGTTCAGGCTCCAGTTCAGG-3′ | NM_001170768.1 |
| Cyclin D1 | F: 5′-GCGAGGAACAGAAGTGCG-3′ R: 5′-TGGAGTTGTCGGTGTAGATGC-3′ | XM_021082686.1 |
| Cyclin E1 | F: 5′-CTCGCCACTGCCTATACTGA-3′ R: 5′-GGTGCCGCTGCATAAGGT-3′ | XM_005653265.2 |
| CDK-4 | F: 5′-GCGGAGATTGGTGTTGGTG-3′ R: 5′-CATTGGGGACTCTTACGCTCTT-3′ | NM_001123097.1 |
| Claudin 1 | F: 5′- GCAGCAGCTTCTTGCTTCTC-3′ R: 5′-CTGGCATTGACTGGGGTCAT-3′ | NM_001244539.1 |
| Occludin | F: 5′-ATCAACAAAGGCAACTCT -3′ R: 5′-GCAGCAGCCATGTACTCT -3′ | XM_005672525.3 |
| ZO-1 | F: 5′-GAGTTTGATATGGGCGTT -3′ R: 5′-GTGGGAGGATGCTGTTGT -3′ | XM_021098896.1 |
| GAPDH | F: 5′-ACAGGGTGGTGGACCTCATG -3′ R: 5′-GGGTCTGGGATGGAAACTGG -3′ | XM_021091114.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yuan, Q.; Wang, T.; Zhan, Y.; Yang, L.; Fan, Y.; Lei, H.; Su, J. Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells. Toxins 2021, 13, 863. https://doi.org/10.3390/toxins13120863
Li Q, Yuan Q, Wang T, Zhan Y, Yang L, Fan Y, Lei H, Su J. Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells. Toxins. 2021; 13(12):863. https://doi.org/10.3390/toxins13120863
Chicago/Turabian StyleLi, Qing, Qiaoling Yuan, Tianjie Wang, Yang Zhan, Lingchen Yang, Ying Fan, Hongyu Lei, and Jianming Su. 2021. "Fumonisin B1 Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells" Toxins 13, no. 12: 863. https://doi.org/10.3390/toxins13120863




