Characterization and Lifetime Dietary Risk Assessment of Eighteen Pyrrolizidine Alkaloids and Pyrrolizidine Alkaloid N-Oxides in New Zealand Honey
Abstract
:1. Introduction
2. Results
2.1. Occurrence of Pyrrolizidine Alkaloids in New Zealand Honey
2.1.1. 2013/2014. Survey
2.1.2. 2016/2017. Survey
2.1.3. 2017/2018. Survey
2.1.4. 2018/2019. Survey
2.1.5. 2019/2020. Survey
2.2. Spatial Variation in Pyrrolizidine Alkaloids in Honey
2.3. Botanical Origin of Pyrrolizidine Alkaloids in Honey
2.4. Exposure Assessment
3. Discussion
3.1. Occurrence of Pyrrolizidine Alkaloids in Honey
3.2. Botanical Profiles
3.3. Linking Regional and Botanical Profiles and Seasonality
3.4. Risk Characterization
3.5. Uncertainty
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. Analytical Testing
5.3. Exposure Assessment Model
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.W.; Culvenor, C.C. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- World Health Organization (WHO) and Food and Agriculture Organization of the United Nations (FAO). Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Eightieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives; Supplement 2: Pyrrolizidine alkaloids; WHO and FAO: Geneva, Switzerland, 2020. [Google Scholar]
- Xia, Q.; He, X.; Shi, Q.; Lin, G.; Fu, P.P. Quantitation of DNA reactive pyrrolic metabolites of senecionine—A carcinogenic pyrrolizidine alkaloid by LC/MS/MS analysis. J. Food Drug Anal. 2020, 28, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, J.A.; Colegate, S.M.; Boppré, M.; Molyneux, R.J. Pyrrolizidine alkaloids in food: A spectrum of potential health consequences. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 308–324. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Report of the 14th session of the Codex Committee on Contaminants in Foods (Virtual) 3–7 and 13 May 2021 REP21/CF; Codex Alimentarius Commission: Rome, Italy, 2021; p. 26. [Google Scholar]
- Commission Regulation. Commission Regulation (EU) 2020/2040 of 11 December 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of pyrrolizidine alkaloids in certain foodstuffs. OJ L 2020, 420, 1–4. [Google Scholar]
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Schedule 23—Prohibited Plants and Fungi; Food Standards Australia New Zealand: Canberra, Australia, 2017.
- United Kingdom Food Standards Agency. Food Business Guidance—Plant Toxins. Available online: https://www.food.gov.uk/business-guidance/plant-toxins (accessed on 25 August 2021).
- Hungerford, N.L.; Carter, S.J.; Anuj, S.R.; Tan, B.L.L.; Hnatko, D.; Martin, C.L.; Sharma, E.; Yin, M.; Nguyen, T.T.P.; Melksham, K.J.; et al. Analysis of Pyrrolizidine Alkaloids in Queensland Honey: Using Low Temperature Chromatography to Resolve Stereoisomers and Identify Botanical Sources by UHPLC-MS/MS. Toxins 2019, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- Lucchetti, M.A.; Glauser, G.; Kilchenmann, V.; Dübecke, A.; Beckh, G.; Praz, C. Pyrrolizidine alkaloids from Echium vulgare in honey originate primarily from floral nectar. J. Agric. Food Chem. 2016, 64, 5267–5273. [Google Scholar] [CrossRef]
- Picron, J.F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Monitoring of pyrrolizidine alkaloids in beehive products and derivatives on the Belgian market. Environ. Sci. Pollut. Res. 2020, 27, 5693–5708. [Google Scholar] [CrossRef]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Bieri, K.; Zoller, O. Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread. Molecules 2019, 24, 2214. [Google Scholar] [CrossRef] [Green Version]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Codex Alimentarius Commission. Code of Practice for Weed Control to Prevent and Reduce Pyrrolizidine Alkaloid Contamination in Food and Feed; CAC/RCP 74–2014; Codex Alimentarius Commission: Rome, Italy, 2014. [Google Scholar]
- Betteridge, K.; Cao, Y.; Colegate, S.M. Improved Method for Extraction and LC-MS Analysis of Pyrrolizidine Alkaloids and Their N-Oxides in Honey: Application to Echium vulgare Honeys. J. Agric. Food Chem. 2005, 53, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Wittig, M.; Reinhard, A.; von der Ohe, K.; Blacquière, T.; Raezke, K.P.; Michel, R.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in honey: Comparison of analytical methods. Food Addit. Contam. Part A Chem Anal. Control. Expo. Risk Assess. 2011, 28, 332–347. [Google Scholar] [CrossRef] [Green Version]
- New Zealand Plant Conservation Network. Available online: https://www.nzpcn.org.nz/ (accessed on 25 August 2021).
- Ngā Tipu o Aotearoa—New Zealand Plants. Available online: https://nzflora.landcareresearch.co.nz/ (accessed on 25 August 2021).
- INaturalist. Available online: https://www.inaturalist.org/ (accessed on 25 August 2021).
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods Discussion Paper on Pyrrolizidine Alkaloids CX/CF 11/5/14; Codex Alimentarius Commission: Rome, Italy, 2011; pp. 37–48. [Google Scholar]
- Langel, L.; Ober, D.; Pelser, P.B. The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem Rev. 2011, 10, 3–74. [Google Scholar] [CrossRef]
- Australia New Zealand Food Authority (ANZFA). Pyrrolizidine Alkaloids in Food. In A Toxicological Review and Risk Assessment; Technical Report Series No. 2; Food Standards Australia New Zealand: Canberra, Australia, 2001. Available online: http://www.foodstandards.gov.au/_srcfiles/TR2.pdf (accessed on 25 August 2021).
- Bundesinstitut für Risikobewertung (Federal Institute for Risk Assessment, BfR). Analytik and Toxizität von Pyrrolizidinalkaloiden Sowie Eine Einschätzung des Gesundheitlichen Risikos Durch Deren Vorkommen in Honig; BfR Opinion No 038/2011; BfR: Berlin, Germany, 2011.
- Committee on Toxicity of Chemicals in Food, Consumer, Products and the Environment (COT). Statement on Pyrrolizidine Alkaloids in Food; COT: London, UK, 2008. [Google Scholar]
- Dübecke, A.; Beckh, G.; Lüllmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A 2011, 28, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Wong, L.; He, Y.; Zhao, Z.; Ye, Y.; Fu, P.P.; Lin, G. Contamination of hepatotoxic pyrrolizidine alkaloids in retail honey in China. Food Control 2018, 85, 484–494. [Google Scholar] [CrossRef]
- Gottschalk, C.; Kaltner, F.; Zimmermann, M.; Korten, R.; Morris, O.; Schwaiger, K.; Gareis, M. Spread of Jacobaea vulgaris and Occurrence of Pyrrolizidine Alkaloids in Regionally Produced Honeys from Northern Germany: Inter- and Intra-Site Variations and Risk Assessment for Special Consumer Groups. Toxins 2020, 12, 441. [Google Scholar] [CrossRef]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Influence of Storage on the Stability of Toxic Pyrrolizidine Alkaloids and Their N-Oxides in Peppermint Tea, Hay, and Honey. J. Agric. Food Chem. 2018, 66, 5221–5228. [Google Scholar] [CrossRef]
- Gottschalk, C.; Huckauf, A.; Dübecke, A.; Kaltner, F.; Zimmermann, M.; Rahaus, I.; Till Beuerle, T. Uncertainties in the determination of pyrrolizidine alkaloid levels in naturally contaminated honeys and comparison of results obtained by different analytical approaches. Food Addit. Contam. Part A 2018, 35, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Burzynski, E.A.; Minbiole, K.P.C.; Livshultz, T. New sources of lycopsamine-type pyrrolizidine alkaloids and their distribution in Apocynaceae. Biochem. Syst. Ecol. 2015, 59, 331–339. [Google Scholar] [CrossRef]
- Apiculture New Zealand. PA Plants—What to Look for and What to Do about it—An Information Pack for Beekeepers; Apiculture New Zealand: Wellington, New Zealand. Available online: https://apinz.org.nz/wp-content/uploads/2021/06/Apiculture-NZ_plant-pack_Final.pdf (accessed on 26 August 2021).
- Pearson, A.; Gibbs, M.; Lau, K.; Edmonds, J.; Alexander, D.; Nicolas, J. The 2016 New Zealand Total Diet Study; MPI: Wellington, New Zealand, 2018; p. 18. [Google Scholar]
- EFSA. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar] [CrossRef] [Green Version]
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicine. Toxicol Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhao, Y.; Von Tungeln, L.S.; Doerge, D.R.; Lin, G.; Cai, L.; Fu, P.P. Pyrrolizidine alkaloid-derived DNA adducts as a common biological biomarker of pyrrolizidine alkaloid-induced tumorigenicity. Chem. Res. Toxicol. 2013, 26, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- MOH (Ministry of Health). NZ Food, NZ Children: Key results of the 2002 National Children’s Nutrition Survey; MOH: Wellington, New Zealand, 2003.
- University of Otago & MOH. A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adults Nutrition Survey; MOH: Wellington, New Zealand, 2011. [Google Scholar]
- MOH. Food and Nutrition Guidelines for Healthy Infants and Toddlers (Aged 0–2): A Background Paper—Partially Revised December 2012; MOH: Wellington, New Zealand, 2008; p. 118.
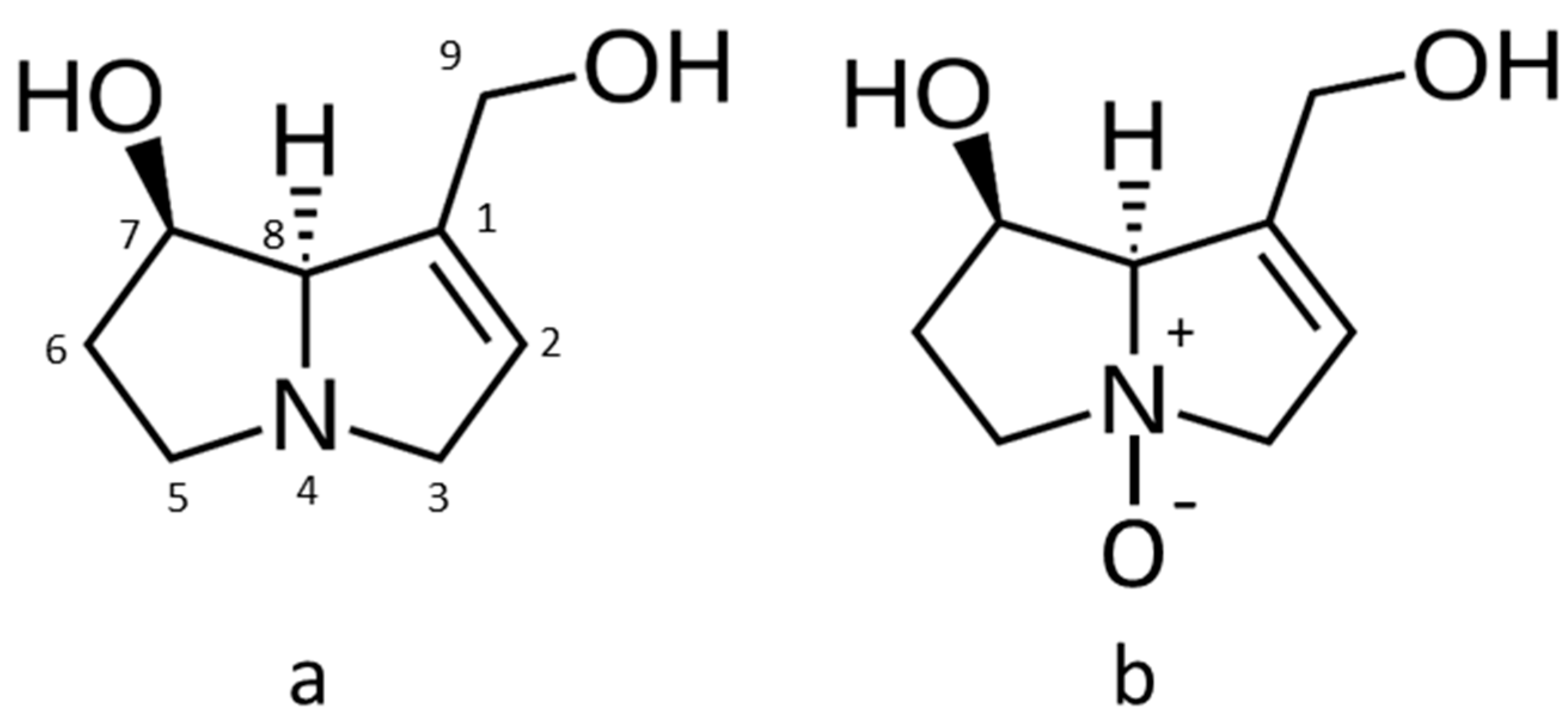


| Survey Year | Honey Type | Sample Number | Samples < LOR Total PA (%) 1 | Total PA Concentration (µg/kg) | Predominant PA Types 2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th% Ile | Median | Mean | 95th% Ile | Maximum | |||||
| 2013/2014 | Drum | 122 | 12 (10) | 0 | 12 | 62 | 350 | 810 | L > E > R > S |
| 2016/2017 | Drum | 65 | 2 (3) | 1 | 7 | 16 | 63 | 130 | L > R > S |
| 2017/2018 | Drum | 255 | 16 (6) | 0 | 12 | 47 | 219 | 641 | L > R > S |
| 2018/2019 | Drum | 339 | 43 (13) | 0 | 12 | 56 | 244 | 2277 | L > S > E > R |
| 2019/2020 | Tank | 274 | 1 (0) | 8 | 46 | 74 | 199 | 912 | L > E > R/S |
| Plant Family | PA Type | Species in New Zealand |
|---|---|---|
| Asteraceae | L | Eupatorium cannabinum (Hemp-agrimony) |
| R/S | Brachyglottis repanda (Rangiora) Erechtites hieraciifolia (American fireweed) Jacobaea vulgaris (Ragwort) Senecio bipinnatisectus (Australian fireweed) Senecio biserratus (Fireweed) * Senecio skirrhodon (Gravel groundsel) # Senecio vulgaris (Common groundsel) | |
| Boraginacaea | E | Echium plantagineum (Patterson’s curse) Echium vulgare (Viper’s bugloss) |
| L | Amsinckia calycina (Yellow gromwell) † Cynoglossum amabile (Chinese forget-me-not) Borago officianalis (Common borage) Myosotis arvensis (Field forget-me-not) Symphytum officinale (Comfrey) Symphytum x uplandicum (Nyman Russian comfrey) |
| Pyrrolizidine Alkaloid | Pyrrolizidine Alkaloid Concentration in Apocynaceae Species Flower Heads (mg/kg dw) | |
|---|---|---|
| Parsonsia heterophylla | Parsonsia capsularis | |
| Intermedine | 22 | 310 |
| Intermedine N-oxide | 3600 | 59,000 |
| Sum of Lycopsamine and Indicine | 1000 | <1 |
| Lycopsamine N-oxide | 51,000 | 520 |
| Senecionine | 0.044 | <1 |
| Survey | Mean Exposure (ng/kg bw/day) | Mean Exposure (MOE) | Median Exposure (ng/kg bw/day) | Median Exposure (MOE) | 95th Percentile Exposure (ng/kg bw/day) | 95th Percentile (MOE) | Percentile of Exposure at Which MOE < 10,000 |
|---|---|---|---|---|---|---|---|
| 2019/2020 | 4.6 | 39,400 | 4.6 | 39,600 | 5.4 | 33,200 | n/a |
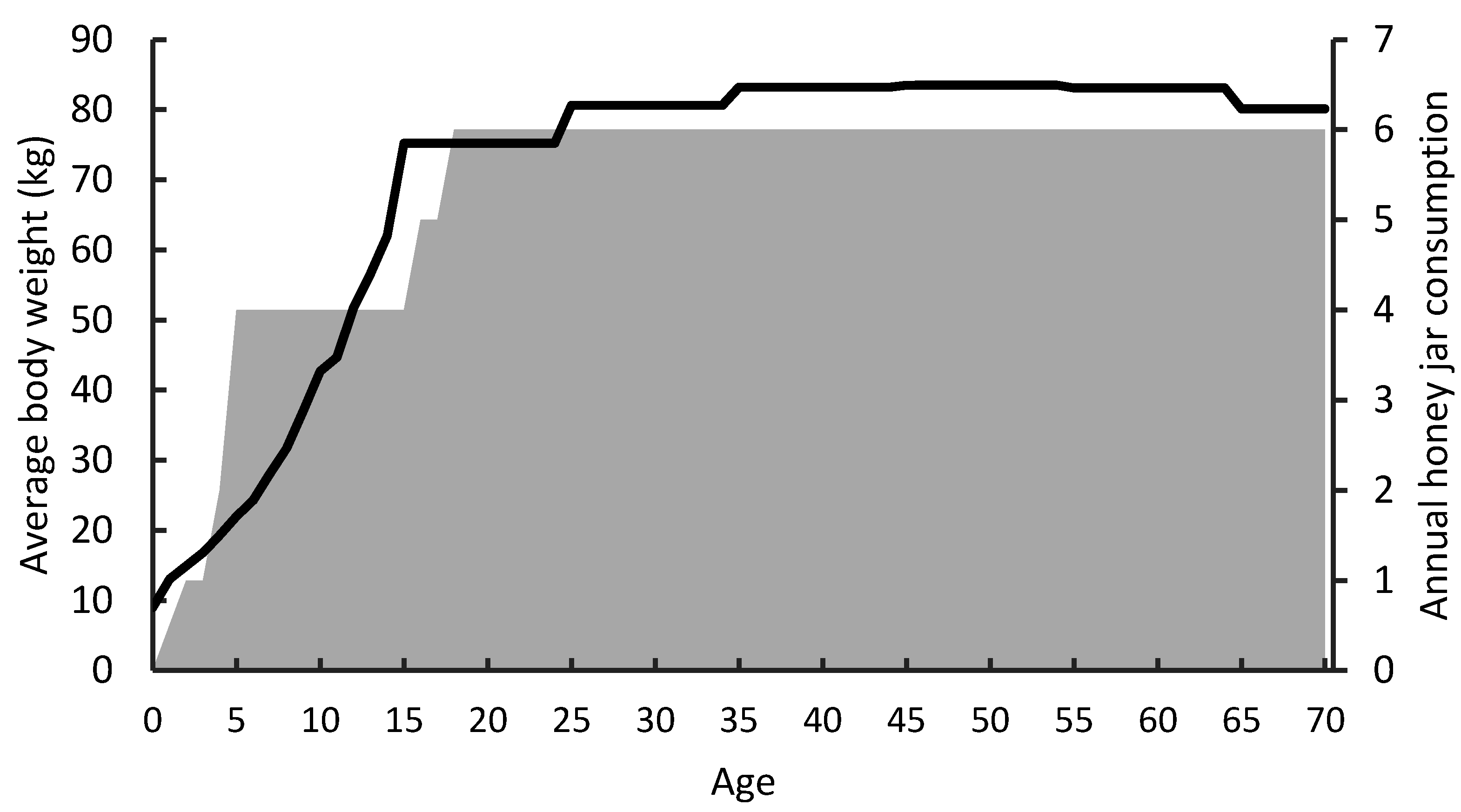
| Age/Gender (Years Old) | Average Bodyweight (kg) | Mean Exposure (ng/kg bw/day) | Median Exposure (ng/kg bw/day) | 95th Percentile Exposure (ng/kg bw/day) | Percentile of Exposure at Which MOE < 10,000 |
|---|---|---|---|---|---|
| 5 | 23 | 11 | 8.5 | 26 | 86th |
| 15 | 54 | 4.6 | 3.7 | 11 | 99th |
| Female (18+) | 70 | 4.9 | 4.2 | 12 | 99th |
| Male (18+) | 82 | 4.2 | 3.7 | 10 | n/a |
| PA Type | Region | Habitat/Practice | Seasonality |
|---|---|---|---|
| Echimidine | South Island high country and Central Otago | Wild fields and gardens of Echium species | Not applicable for these regions |
| Lycopsamine | Wairarapa and Manawatu (Lower North Island) | Bush margins that contain Parsonsia vines Wild or commercial sites, and gardens containing borage, comfrey, and other Boraginaceae and Eupatorieae | Not applicable for these regions |
| Retrorsine/Senecionine | Northland, Coromandel Peninsula and East Cape | Forestry blocks felled in past 5 years Burnt, cleared, or barren land Weed infested pasture | Second harvest samples had a higher frequency of higher levels of PAs |
| Action | Uncertainty | Consequence |
|---|---|---|
| Use of total PA values in risk assessment. | Relative potency of the toxicity of different PAs. | Major: Overestimate of toxicity by potential 1000-fold. |
| Use of lower bound approach (ND = zero). | Assumes absence of PAs when not detected. | Minor: Few results are fully ND; NDs are most common for PAs with minimal profile in NZ honey, e.g., riddelliine. |
| Use of a suite of 18 PAs and N-oxide | Potential occurrence of other PA congeners in the samples. Masked PAs from the loss of PA-N-oxides during processing and storage. | Moderate: Exposure could be underestimated if other PAs were notable. However, the tested suite aligns with PA testing recommendations in overseas studies [34]. |
| Modelling consumption practices based only on 500 g retail jars. | ~20% of honey is retailed in 250 g jars, other sizes up to 1 kg also sold. | Minor: Other jar sizes are a smaller proportion of retail; analysis of 250 g vs. 500 g shows limited impact on exposure. |
| Modelling lifetime exposure based on daily honey consumption amounts. | Minimal information available on long-term honey consumption practice. | Moderate: Exposure could be under/over-estimated in the population. Very high honey consumers (e.g., beekeepers) difficult to capture. |
| Weighting retail honey survey dataset based on market share. | 10% of retailed honey not captured. Does not account for brand/honey type loyalty. Assumes nationwide distribution of all honey. | Moderate: Likely underestimates exposure for consumers with brand/type loyalty. |
| Consideration only of honey contribution to dietary PA exposure. | Presence of PAs, and dietary exposures to PAs from other foods available to NZ consumers is unknown. | Moderate: Risk characterization conclusion could be underestimated. |
| Pyrrolizidine Alkaloid | 2013/2014 | 2016/2017 | 2017/2018 | 2018/2019 | 2019/2020 |
|---|---|---|---|---|---|
| Echimidine | Tested | Tested | Tested | Tested | Tested |
| Echimidine-N-Oxide | Tested | Tested | Tested | Tested | Tested |
| Echivulgarine | Tested | Tested | - | - | - |
| Echivulgarine N-oxide | Tested | Tested | - | - | - |
| Intermedine | - | - | Tested 1 | Tested 1 | Tested 1 |
| Intermedine-N-oxide | - | - | Tested 2 | Tested 2 | Tested 2 |
| Indicine | - | - | Tested 1 | Tested 1 | Tested 1 |
| Lasiocarpine | - | - | Tested | Tested | Tested |
| Lasiocarpine-N-oxide | - | - | Tested | Tested | Tested |
| Lycopsamine | Tested | Tested | Tested 1 | Tested 1 | Tested 1 |
| Lycopsamine-N-oxide | Tested | - | Tested 2 | Tested 2 | Tested 2 |
| Retrorsine | Tested | Tested | Tested | Tested | Tested |
| Retrorsine-N-oxide | Tested | Tested | Tested | Tested | Tested |
| Riddelliine | - | - | - | Tested 3 | Tested |
| Senecionine | Tested | Tested | Tested 4 | Tested 4 | Tested 4 |
| Senecionine-N-oxide | Tested | Tested | Tested 5 | Tested 5 | Tested 5 |
| Seneciphylline | Tested | Tested | Tested | Tested | Tested |
| Seneciphylline-N-oxide | Tested | Tested | Tested | Tested | Tested |
| Senecivernine | - | - | Tested 4 | Tested 4 | Tested 4 |
| Senecivernine-N-oxide | - | - | Tested 5 | Tested 5 | Tested 5 |
| Senkirkine | Tested | Tested | Tested | Tested | Tested |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearson, A.J.; Nicolas, J.E.F.; Lancaster, J.E.; Symes, C.W. Characterization and Lifetime Dietary Risk Assessment of Eighteen Pyrrolizidine Alkaloids and Pyrrolizidine Alkaloid N-Oxides in New Zealand Honey. Toxins 2021, 13, 843. https://doi.org/10.3390/toxins13120843
Pearson AJ, Nicolas JEF, Lancaster JE, Symes CW. Characterization and Lifetime Dietary Risk Assessment of Eighteen Pyrrolizidine Alkaloids and Pyrrolizidine Alkaloid N-Oxides in New Zealand Honey. Toxins. 2021; 13(12):843. https://doi.org/10.3390/toxins13120843
Chicago/Turabian StylePearson, Andrew J., Jeane E. F. Nicolas, Jane E. Lancaster, and C. Wymond Symes. 2021. "Characterization and Lifetime Dietary Risk Assessment of Eighteen Pyrrolizidine Alkaloids and Pyrrolizidine Alkaloid N-Oxides in New Zealand Honey" Toxins 13, no. 12: 843. https://doi.org/10.3390/toxins13120843





