Identification and Structure Elucidation of Epoxyjanthitrems from Lolium perenne Infected with the Endophytic Fungus Epichloë festucae var. lolii and Determination of the Tremorgenic and Anti-Insect Activity of Epoxyjanthitrem I
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidatation
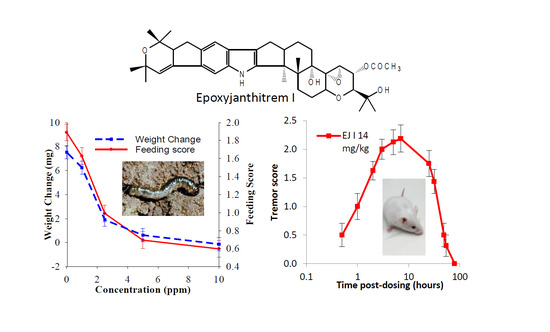
2.2. Insect Bioassay
2.3. Mouse Bioassay
3. Conclusions
4. Materials and Methods
4.1. Epoxyjanthitrem Analysis by Analytical HPLC
4.2. Isolation of Epoxyjanthitrem I for Testing on Insects and Mice
4.3. Insect Bioassay
4.4. Mouse Bioassay
4.5. Isolation of Epoxyjanthitrems for Structural Elucidation
4.6. Mass Spectrometry
4.7. NMR Spectroscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Hunt, M.B.; Gaynor, D.L. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte Acremonium loliae. J. Chem. Soc. Chem. Commun. 1986, 12, 935–936. [Google Scholar] [CrossRef]
- Ball, O.J.P.; Miles, C.O.; Prestidge, R.A. Ergopeptine alkaloids and Neotyphodium lolii-mediated resistance in perennial ryegrass against adult Heteronychus arator (Coleoptera: Scarabaeidae). J. Econ. Entomol. 1997, 90, 1382–1391. [Google Scholar] [CrossRef]
- di Menna, M.E.; Finch, S.C.; Popay, A.J.; Smith, B.L. A review of the Neotyphodium lolii/Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. N. Z. Vet. J. 2012, 60, 315–328. [Google Scholar] [CrossRef]
- Klotz, J. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 2015, 7, 2801–2821. [Google Scholar] [CrossRef]
- Johnson, L.J.; De Bonth, A.C.M.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Fletcher, L.R. “Non-toxic” endophytes in ryegrass and their effect on livestock health and production. Grassl. Res. Pract. Ser. 1999, 7, 133–139. [Google Scholar]
- Popay, A.J.; Cox, N.R. Aploneura lentisci (Homoptera: Aphididae) and its interactions with fungal endophytes in perennial ryegrass (Lolium perenne). Front. Plant Sci. 2016, 7, 1395. [Google Scholar] [CrossRef] [Green Version]
- Popay, A.J.; Hume, D.E.; Baltus, J.G.; Latch, G.C.M.; Tapper, B.A.; Lyons, T.B.; Cooper, B.M.; Pennell, C.G.; Eerens, J.P.J.; Marshall, S.L. Field performance of perennial ryegrass (Lolium perenne) infected with toxin-free fungal endophytes (Neotyphodium spp.). Ryegrass Endophyte Essent. N. Z. Symbiosis Grassl. Res. Pract. Ser. 1999, 7, 113–122. [Google Scholar]
- Popay, A.J.; Wyatt, R.T. Resistance to Argentine stem weevil in perennial ryegrass infected with endophytes producing different alkaloids. In Proceedings of the Forty Eighth New Zealand Plant Protection Conference, Hastings, New Zealand, 8–10 August 1995; Volume 48, pp. 229–236. [Google Scholar]
- Popay, A.J.; Thom, E.R. Endophyte effects on major insect pests in Waikato dairy pasture. Proc. N. Z. Grassl. Assoc. 2009, 71, 121–126. [Google Scholar]
- Jensen, J.G.; Popay, A.J. Perennial ryegrass infected with AR37 endophyte reduces survival of porina larvae. N. Z. Plant Prot. 2004, 57, 323–328. [Google Scholar] [CrossRef]
- Popay, A.J.; Cotching, B.; Moorhead, A.; Ferguson, C.M. AR37 reduces porina populations and plant damage in the field. Proc. N. Z. Grassl. Assoc. 2012, 74, 165–169. [Google Scholar]
- Popay, A.J.; Gerard, P.J. Cultivar and endophyte effects on a root aphid, Aploneura lentisci, in perennial ryegrass. N. Z. Plant Prot. 2007, 60, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Hume, D.E.; Ryan, D.L.; Cooper, B.M.; Popay, A.J. Agronomic performance of AR37-infected ryegrass in northern New Zealand. Proc. N. Z. Grassl. Assoc. 2007, 69, 201–205. [Google Scholar] [CrossRef]
- Pennell, C.G.L.; Popay, A.J.; Ball, O.J.P.; Hume, D.E.; Baird, D.B. Occurrence and impact of pasture mealybug (Balanococcus poae) and root aphid (Aploneura lentisci) on ryegrass (Lolium spp.) with and without infection by Neotyphodium fungal endophytes. N. Z. J. Agric. Res. 2005, 48, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, L.R. Managing ryegrass-endophyte toxicoses. In Neotyphodium in Cool-Season Grasses; Roberts, C.A., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; pp. 229–241. [Google Scholar]
- Caradus, J.R.; Johnson, L.J. Improved adaptation of temperate grasses through mutualism with fungal endophytes. In Endophyte biotechnology: Promise for agriculture and pharmacology; Schouten, A., Ed.; CAB International: Wallingford, UK, 2019; pp. 85–108. [Google Scholar]
- Ludlow, E.J.; Vassiliadis, S.; Ekanayake, P.N.; Hettiarachchige, I.K.; Reddy, P.; Sawbridge, T.I.; Rochfort, S.J.; Spangenberg, G.C.; Guthridge, K.M. Analysis of the indole diterpene gene cluster for biosynthesis of the epoxy-janthitrems in Epichloë endophytes. Microorganisms 2019, 7, 560. [Google Scholar] [CrossRef] [Green Version]
- Finch, S.C.; Fletcher, L.R.; Babu, J.V. The evaluation of endophyte toxin residues in sheep fat. N. Z. Vet. J. 2012, 60, 56–60. [Google Scholar] [CrossRef]
- Finch, S.C.; Thom, E.R.; Babu, J.V.; Hawkes, A.D.; Waugh, C.D. The evaluation of fungal endophyte toxin residues in milk. N. Z. Vet. J. 2013, 61, 11–17. [Google Scholar] [CrossRef]
- Tapper, B.A.; Cooper, B.M.; Easton, H.S.; Fletcher, L.R.; Hume, D.E.; Lane, G.A.; Latch, G.C.M.; Pennell, C.G.L.; Popay, A.J.; Christensen, M.J. Improvements in Grass Endophytes. WO Patent 2004/106487, 3 June 2004. [Google Scholar]
- Moate, P.J.; Williams, S.R.O.; Grainger, C.; Hannah, M.C.; Mapleson, D.; Auldist, M.J.; Greenwood, J.S.; Popay, A.J.; Hume, D.E.; Mace, W.J.; et al. Effects of wild-type, AR1 and AR37 endophyte-infected perennial ryegrass on dairy production in Victoria, Australia. Anim. Prod. Sci. 2012, 52, 1117–1130. [Google Scholar] [CrossRef]
- Babu, J.V.; Popay, A.J.; Miles, C.O.; Wilkins, A.L.; di Menna, M.E.; Finch, S.C. Identification and Structure Elucidation of Janthitrems A and D from Penicillium janthinellum and Determination of the Tremorgenic and Anti-Insect Activity of Janthitrems A and B. J. Agric. Food Chem. 2018, 66, 13116–13125. [Google Scholar] [CrossRef]
- Penn, J.; Swift, R.; Wigley, L.J.; Mantle, P.G.; Bilton, J.N.; Sheppard, R.N. Janthitrems B and C, two principal indole-diterpenoids produced by Penicillium janthinellum. Phytochemistry 1993, 32, 1431–1434. [Google Scholar] [CrossRef]
- Hennessy, L.M.; Popay, A.J.; Finch, S.C.; Clearwater, M.J.; Cave, V.M. Temperature and Plant Genotype Alter Alkaloid Concentrations in Ryegrass Infected with an Epichloë Endophyte and This Affects an Insect Herbivore. Front. Plant Sci. 2016, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Gallagher, R.T.; Hawkes, A.D.; Munday, S.C.; Towers, N.R. Synthesis and tremorgenicity of paxitriols and lolitriol: Possible biosynthetic precursors of Lolitrem B. J. Agric. Food Chem. 1992, 40, 234–238. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Miles, C.O.; Ede, R.M.; Gallagher, R.T.; Munday, S.C. Structure elucidation of janthitrem B, a tremorgenic metabolite of Penicillium janthinellum, and relative configuration of the A and B rings of janthitrems B, E, and F. J. Agric. Food Chem. 1992, 40, 1307–1309. [Google Scholar] [CrossRef]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O. Isolation of lolicine A, lolicine B, lolitriol, and lolitrem N from Lolium perenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-epilolitrem N and 31-epilolitrem F. J. Agric. Food Chem. 1998, 46, 590–598. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Hawkes, A.D.; Steyn, P.S.; Vleggaar, R. Tremorgenic Neurotoxins from Perennial Ryegrass causing Ryegrass Staggers Disorder of Livestock: Structure Elucidation of Lolitrem B. J. Chem. Society Chem. Commun. 1984, 9, 614–616. [Google Scholar] [CrossRef]
- Miles, C.O.; Munday, S.C.; Wilkins, A.L.; Ede, R.M.; Towers, N.R. Large-scale isolation of lolitrem B and structure determination of lolitrem E. J. Agric. Food Chem. 1994, 42, 1488–1492. [Google Scholar] [CrossRef]
- Fletcher, L.R.; Finch, S.C.; Sutherland, B.L.; deNicolo, G.; Mace, W.J.; van Koten, C.; Hume, D.E. The occurrence of ryegrass staggers and heat stress in sheep grazing ryegrass-endophyte associations with diverse alkaloid profiles. N. Z. Vet. J. 2017, 65, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, R.T.; Latch, G.C.M.; Keogh, R.G. The janthitrems: Fluorescent tremorgenic toxins produced by Penicillium janthinellum isolates from ryegrass pastures. Appl. Environ. Microbiol. 1980, 39, 272–273. [Google Scholar] [CrossRef] [Green Version]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O.; Ede, R.M.; Thomson, R.A. Structure elucidation of lolitrem F, a naturally-occurring stereoisomer of the tremorgenic mycotoxin lolitrem B, isolated from Lolium perenne infected with Acremonium lolii. J. Agric. Food Chem. 1996, 44, 2782–2788. [Google Scholar] [CrossRef]
- Huang, X.-H.; Nishida, H.; Tomoda, H.; Tabata, N.; Shiomi, K.; Yang, D.-J.; Takayanagi, H.; Omura, S. Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis II. Structure elucidation of terpendoles A, B, C and D. J. Antibiot. 1995, 43, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.-H.; Tomoda, H.; Nishida, H.; Masuma, R.; Omura, S. Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis I. Production, isolation and biological properties. J. Antibiot. 1995, 43, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O.; Tomoda, H.; Omura, S. Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins. J. Agric. Food Chem. 1997, 45, 199–204. [Google Scholar] [CrossRef]
- Thom, E.R.; Waugh, C.D.; Minnee, E.M.K.; Waghorn, G.C. A new generation ryegrass endophyte—The first results from dairy cows fed AR37. NZGA Res. Pract. Ser. 2006, 13, 293–296. [Google Scholar]
- Popay, A.J. A laboratory method for rearing porina. N. Z. Plant Prot. 2001, 54, 251. [Google Scholar] [CrossRef]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O. Isolation of paspaline B, an indole–diterpenoid from Penicillium paxilli. Phytochemistry 1996, 41, 327–332. [Google Scholar] [CrossRef]
- Springer, J.P.; Clardy, J.; Wells, J.M.; Cole, R.J.; Kirksey, J.W. The structure of paxilline, a tremorgenic metabolite of Penicillium paxilli Bainier. Tetrahedron Lett. 1975, 30, 2531–2534. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Hawkes, A.D. The potent tremorgenic neurotoxins lolitrem B and aflatrem: A comparison of the tremor response in mice. Experientia 1986, 42, 823–825. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Hawkes, A.D. Estimation of neurotoxin levels in perennial ryegrass by mouse bioassay. N. Z. J. Agric. Res. 1985, 28, 427–431. [Google Scholar] [CrossRef]



| Atom | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 9.86, s a | 9.87 s | 9.83, s | 9.86, s | 9.84, s |
| 5 | 2.68, m | 2.64, m | 2.65, m | 2.67, m | 2.65, m |
| 1.66, m | 1.63, m | 1.64, m | 1.66, m | 1.64, m | |
| 6 | 2.28, m | 2.18, m | 2.21 m, | 2.26, m | 2.25, m |
| 1.83, m | 1.77, m | 1.78, m | 1.79, m | 1.80, m | |
| 7 | 4.20, t (8.9) | 4.32, t (9.0) | 4.18, t (8.9) | 4.16, t (9.0) | 4.19, t (9.0) |
| 9 | 3.48, d (9.4) | 3.50, d (9.3) | 3.39, d (9.1) | 3.59, d (9.3) | 3.35, d (9.0) |
| 10 | 5.17, d (9.4) | 4.04, d (9.3) | 3.95, d (9.1) | 5.26, d (9.3) | 3.99, d (9.0) |
| 11 | 3.55, s | 3.56, s | 3.50, s | 3.51, s | 3.52, s |
| 14 | 1.63, m | 1.65, m | 1.61, m | 1.63, m | 1.63, m |
| 1.54, m | 1.53, m | 1.55, m | 1.56, m | 1.54, m | |
| 15 | 1.95, m | 1.95, m | 1.96 m, | 1.98, m | 1.96, m |
| 1.57, m | 1.56, m | 1.58, m | 1.56, m | 1.57, m | |
| 16 | 2.81, m | 2.77, m | 2.81, m | 2.81, m | 2.81, m |
| 17 | 2.65, m | 2.64, m | 2.65, m | 2.63, m | 2.65, m |
| 2.35, dd (10.9, 13.0) | 2.35, dd, (11.09, 12.8) | 2.35, dd (10.9, 12.9) | 2.35, dd (11.0, 13.0) | 2.35, dd (11.1, 12.9) | |
| 20 | 7.18, s | 7.18, s | 7.18, s | 7.17, s | 7.18, s |
| 23 | 7.39 s | 7.38, s | 7.38, s | 7.38, s | 7.39, s |
| 25 | 1.32, s | 1.32, s | 1.32, s | 1.32, s | 1.32, s |
| 26 | 1.16, s | 1.14, s | 1.13, s | 1.17, s | 1.14, s |
| 28 | 1.13, s | 1.18, s | 1.28, s | 1.20, s | 1.19, s |
| 29 | 1.14, s | 1.28, s | 1.25, s | 1.18, s | 1.22, s |
| 30 | 3.08, dd (9.4, 15.6) | 3.09, dd (9.3, 15.8) | 3.09, dd (9.4, 15.6) | 3.08, dd (9.2, 15.5) | 3.09, dd (9.3, 15.6) |
| 2.66, m | 2.64, m | 2.65, m | 2.64, m | 2.65, m | |
| 31 | 2.83, m | 2.83, m | 2.86, m | 2.86, m | 2.87, m |
| 35 | 5.93, d (2.9) | 5.95, d (3.0) | 5.94, d (3.0) | 5.93, d (3.0) | 5.94, d (3.0) |
| 37 | 1.27, s | 1.27, s | 1.27, s | 1.27, s | 1.27, s |
| 38 | 1.05, s | 1.05, s | 1.05, s | 1.05, s | 1.05, s |
| 39 | 1.25, s | 1.24, s | 1.25, s | 1.25, s | 1.25, s |
| 40 | 1.30, s | 1.30, s | 1.30, s | 1.30, s | 1.30, s |
| 42 | 2.05, s | ||||
| 43 | 5.58, d (6.2) | 4.03, dd (2.6, 6.7) | 3.92, d (6.6) | ||
| 44 | 5.17, d (6.2) | 5.26, t (6.7) | 5.20, t (6.6) | ||
| 46 | 1.70, s | 1.71, s | 1.68, s | ||
| 47 | 1.69, s | 1.66, s | 1.63, s | ||
| 49 | 1.99, s | ||||
| 10-OH | 4.29, s | ||||
| 13-OH | 3.29, s | 3.42, s | 3.31, s | ||
| 27-OH |
| Atom | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 154.9 | 154.9 | 155.0 | 154.9 | 155.0 |
| 3 | 51.7 | 51.7 | 51.7 | 51.6 | 51.7 |
| 4 | 43.2 | 43.3 | 43.2 | 43.2 | 43.3 |
| 5 | 26.9 | 27.1 | 27.0 | 26.9 | 27.2 |
| 6 | 28.7 | 29.5 a | 29.1 a | 28.8 | 29.3 a |
| 7 | 72.3 | 72.2 | 72.2 | 72.3 | 72.1 |
| 9 | 76.6 | 72.2 | 75.8 | 75.1 | 77.1 |
| 10 | 68.7 | 71.8 | 68.0 | 67.9 | 68.3 |
| 11 | 62.3 | 60.9 | 64.3 | 62.7 | 64.5 |
| 12 | 70.4 | 68.1 | 69.0 | 70.3 | 69.4 |
| 13 | 78.0 | 78.4 | 78.1 | 77.9 | 78.1 |
| 14 | 30.3 | 30.1 a | 30.4 a | 30.3 a | 30.3 a |
| 15 | 21.5 | 21.5 | 21.5 | 21.5 | 21.5 |
| 16 | 50.9 | 50.9 | 50.9 | 50.9 | 50.9 |
| 17 | 27.8 | 27.7 | 27.8 | 27.7 | 27.8 |
| 18 | 116.8 | 116.7 | 116.8 | 116.8 | 116.8 |
| 19 | 127.7 | 127.4 | 127.7 | 127.7 | 127.7 |
| 20 | 114.3 | 114.3 | 114.3 | 114.3 | 114.3 |
| 21 | 140.9 | 140.8 | 140.9 | 141.3 | 140.9 |
| 22 | 133.6 | 133.5 | 133.5 | 133.5 | 133.5 |
| 23 | 104.0 | 104.0 | 104.0 | 104.0 | 104.0 |
| 24 | 141.3 | 141.3 | 141.3 | 141.3 | 141.3 |
| 25 | 16.5 | 16.4 | 16.5 | 16.4 | 16.5 |
| 26 | 18.8 | 18.8 | 18.7 | 18.8 | 18.7 |
| 27 | 71.4 | 75.0 | 79.5 | 76.8 | 73.2 |
| 28 | 26.7 b | 28.8 | 23.9 | 24.5 | 24.3 |
| 29 | 26.6 b | 17.1 | 19.7 | 21.4 | 28.5 |
| 30 | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 |
| 31 | 49.8 | 49.8 | 49.8 | 49.8 | 49.8 |
| 32 | 74.7 | 74.7 | 74.7 | 74.7 | 74.7 |
| 34 | 72.9 | 72.9 | 72.9 | 72.9 | 72.9 |
| 35 | 119.6 | 119.7 | 119.7 | 119.6 | 119.7 |
| 36 | 136.9 | 136.8 | 136.9 | 136.9 | 136.9 |
| 37 | 30.6 | 30.5 | 30.4 a,b | 30.4 a,b | 30.3 a,b |
| 38 | 22.4 | 22.4 | 22.4 | 22.4 | 22.4 |
| 39 | 32.4 | 32.3 | 32.3 | 32.3 | 32.4 |
| 40 | 30.6 | 30.5 | 30.4 a,b | 30.4 a,b | 30.3 a,b |
| 41 | 170.5 | ||||
| 42 | 21.2 | ||||
| 43 | 93.3 | 58.8 | 59.3 | ||
| 44 | 124.0 | 122.2 | 124.0 | ||
| 45 | 138.1 | 137.0 | 134.4 | ||
| 46 | 25.5 | 25.8 | 25.7 | ||
| 47 | 18.8 | 18.0 | 18.0 | ||
| 48 | 170.2 | ||||
| 49 | 21.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finch, S.C.; Prinsep, M.R.; Popay, A.J.; Wilkins, A.L.; Webb, N.G.; Bhattarai, S.; Jensen, J.G.; Hawkes, A.D.; Babu, J.V.; Tapper, B.A.; et al. Identification and Structure Elucidation of Epoxyjanthitrems from Lolium perenne Infected with the Endophytic Fungus Epichloë festucae var. lolii and Determination of the Tremorgenic and Anti-Insect Activity of Epoxyjanthitrem I. Toxins 2020, 12, 526. https://doi.org/10.3390/toxins12080526
Finch SC, Prinsep MR, Popay AJ, Wilkins AL, Webb NG, Bhattarai S, Jensen JG, Hawkes AD, Babu JV, Tapper BA, et al. Identification and Structure Elucidation of Epoxyjanthitrems from Lolium perenne Infected with the Endophytic Fungus Epichloë festucae var. lolii and Determination of the Tremorgenic and Anti-Insect Activity of Epoxyjanthitrem I. Toxins. 2020; 12(8):526. https://doi.org/10.3390/toxins12080526
Chicago/Turabian StyleFinch, Sarah C., Michèle R. Prinsep, Alison J. Popay, Alistair L. Wilkins, Nicola G. Webb, Sweta Bhattarai, Joanne G. Jensen, Allan D. Hawkes, Jacob V. Babu, Brian A. Tapper, and et al. 2020. "Identification and Structure Elucidation of Epoxyjanthitrems from Lolium perenne Infected with the Endophytic Fungus Epichloë festucae var. lolii and Determination of the Tremorgenic and Anti-Insect Activity of Epoxyjanthitrem I" Toxins 12, no. 8: 526. https://doi.org/10.3390/toxins12080526