NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. NLRP3 Inflammasome Activation in Monocytes of HD and BP Patients
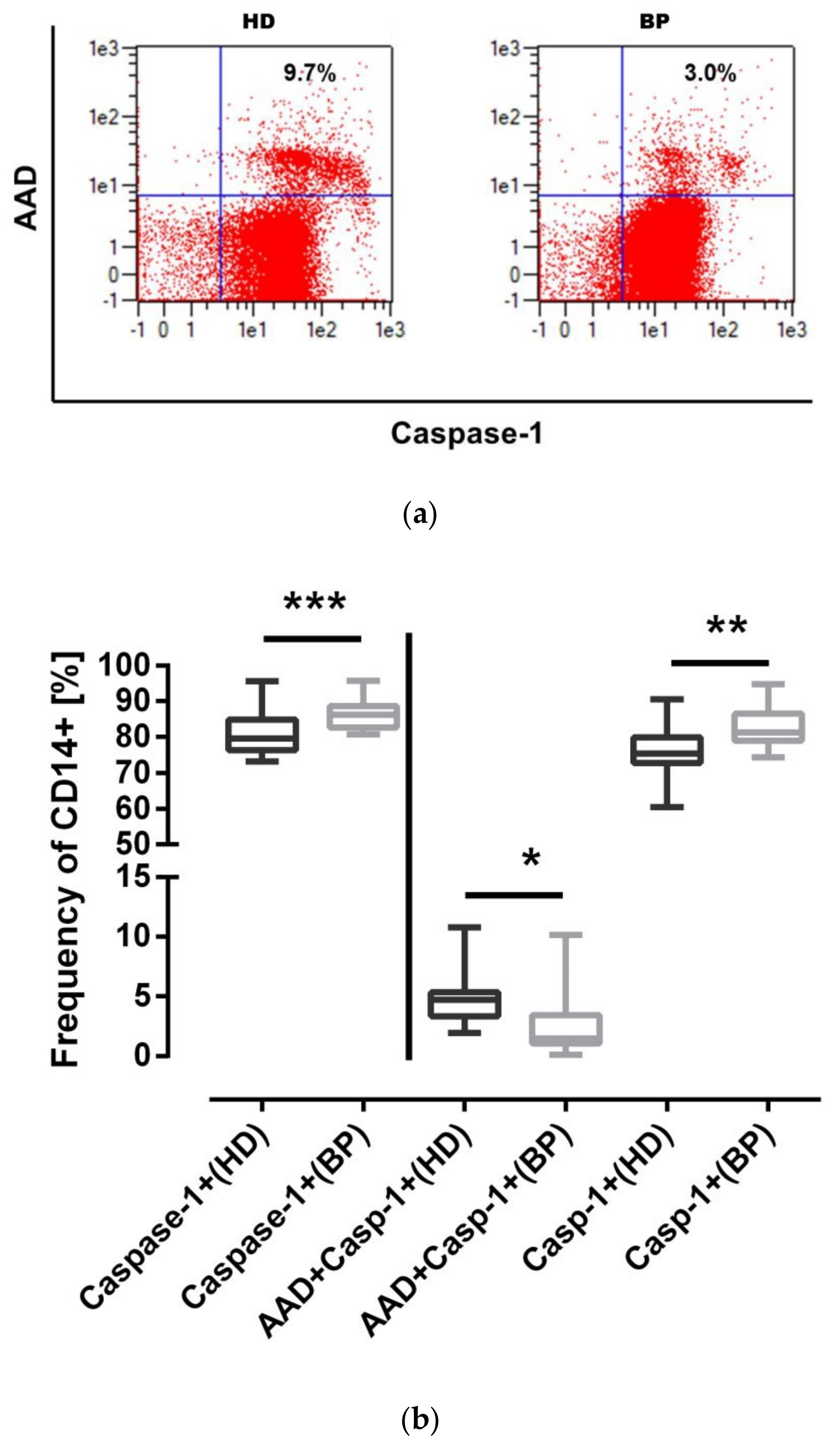
2.3. NLRP3 Inflammasome Activation: The Pyroptotic Route in HD Patients
2.4. IL-1β Expression: Higher Levels of IL-1β in BP Patients
2.5. Full Activation of the NLRP3 Inflammasome: Pyroptosis in HD Patients
2.6. Intracellular ATP Measurement
2.7. Association between BMI and Caspase-1 Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Clinical Procedures
5.3. PBMC Isolation
5.4. NLRP3 Inflammasome Stimulation Model
5.5. Antibodies for Flow Cytometry
5.6. Caspase-1-Assay
5.7. Time of Flight Evaluation of ASC Specks by Flow Cytometry
5.8. Determination of Intracellular ATP
5.9. RNA/cDNA/qPCR
5.10. Simple Western Analysis
5.11. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Wright, J.; Hutchison, A. Cardiovascular disease in patients with chronic kidney disease. Vasc. Health Risk Manag. 2009, 5, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, C.U.; Lee, R.T.; Rifai, N.; Ridker, P.M. Blood pressure and inflammation in apparently healthy men. Hypertension 2001, 38, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.M.; Sobey, C.G.; Latz, E.; Mansell, A.; Drummond, G.R. IL-1β and IL-18: Inflammatory markers or mediators of hypertension? Br. J. Pharmacol. 2014, 171, 5589–5602. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999, 138, S419–S420. [Google Scholar] [CrossRef]
- Sester, D.P.; Thygesen, S.J.; Sagulenko, V.; Vajjhala, P.R.; Cridland, J.A.; Vitak, N.; Chen, K.W.; Osborne, G.W.; Schroder, K.; Stacey, K.J. A novel flow cytometric method to assess inflammasome formation. J. Immunol. 2015, 194, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Chen, C.; Chen, Z.; Liu, L.; Jiang, J.; Wu, Z.; Zhao, M.; Chen, Y. NLRP3: A Novel Mediator in Cardiovascular Disease. J. Immunol. Res. 2018, 2018, 5702103. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2018, 113, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Iturbe, B.; Pons, H.; Quiroz, Y.; Johnson, R.J. The immunological basis of hypertension. Am. J. Hypertens. 2014, 27, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.M.; Coppinger, T.; Waywell, C.; Dunne, L.; Archer, L.D.; Critchley, W.R.; Yonan, N.; Fildes, J.E.; Williams, S.G. The effect of beta-blockers on the adaptive immune system in chronic heart failure. Cardiovasc. Ther. 2009, 27, 181–186. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [Green Version]
- Ahechu, P.; Zozaya, G.; Martí, P.; Hernández-Lizoáin, J.L.; Baixauli, J.; Unamuno, X.; Frühbeck, G.; Catalán, V. NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development. Front. Immunol. 2018, 9, 2918. [Google Scholar] [CrossRef] [PubMed]
- Rogacev, K.S.; Ulrich, C.; Blömer, L.; Hornof, F.; Oster, K.; Ziegelin, M.; Cremers, B.; Grenner, Y.; Geisel, J.; Schlitt, A.; et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 2010, 31, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why primary obesity is a disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [Green Version]
- Gurung, P.; Anand, P.K.; Malireddi, R.K.S.; Vande Walle, L.; van Opdenbosch, N.; Dillon, C.P.; Weinlich, R.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.-D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014, 192, 1835–1846. [Google Scholar] [CrossRef] [Green Version]
- Orning, P.; Lien, E.; Fitzgerald, K.A. Gasdermins and their role in immunity and inflammation. J. Exp. Med. 2019, 216, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, G. Mechanisms and Therapeutic Regulation of Pyroptosis in Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2020, 21, 1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rühl, S.; Shkarina, K.; Demarco, B.; Heilig, R.; Santos, J.C.; Broz, P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 2018, 362, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Nomura, J.; So, A.; Tamura, M.; Busso, N. Intracellular ATP Decrease Mediates NLRP3 Inflammasome Activation upon Nigericin and Crystal Stimulation. J. Immunol. 2015, 195, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [Green Version]
- Carta, S.; Penco, F.; Lavieri, R.; Martini, A.; Dinarello, C.A.; Gattorno, M.; Rubartelli, A. Cell stress increases ATP release in NLRP3 inflammasome-mediated autoinflammatory diseases, resulting in cytokine imbalance. Proc. Natl. Acad. Sci. USA 2015, 112, 2835–2840. [Google Scholar] [CrossRef] [Green Version]
- Piccini, A.; Carta, S.; Tassi, S.; Lasiglié, D.; Fossati, G.; Rubartelli, A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA 2008, 105, 8067–8072. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.A.; Bergstralh, D.T.; Wang, Y.; Willingham, S.B.; Ye, Z.; Zimmermann, A.G.; Ting, J.P.-Y. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 8041–8046. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.-M.; Hu, W.; Troutman, T.D.; Jennings, M.; Brewer, T.; Li, X.; Nanda, S.; Cohen, P.; Thomas, J.A.; Pasare, C. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2014, 111, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Kaczanowska, S.; Davila, E. IL-1 Receptor-Associated Kinase Signaling and Its Role in Inflammation, Cancer Progression, and Therapy Resistance. Front. Immunol. 2014, 5, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| HD (N = 20) | BP (N = 20) | Statistics | |
|---|---|---|---|
| Age (years) | 57.7 ± 14.3 | 58.0 ± 12.2 | 0.864 |
| Gender (female) (%) (n) | 40 (8) | 40 (8) | 1.000 |
| BMI (kg/m2) | 25.9 ± 5.1 | 29.3 ± 5.7 | 0.022 |
| Diabetes mellitus (%) (n) | 10 (2) | 10 (2) | 1.000 |
| Hypertension (%) (n) | 100 (20) | 100 (20) | 1.000 |
| BP (sys., mm Hg) | 144 ± 23 | 147 ± 14 | 0.880 |
| BP (diast., mm Hg) | 84 ± 17 | 85 ± 5 | 0.697 |
| Apoplex (%) (n) | 0 (0) | 5 (1) | 1.000 |
| CHD (%) (n) | 10 (2) | 10 (2) | 1.000 |
| pAD (%) (n) | 0 (0) | 0 (0) | 1.000 |
| MI (%) (n) | 0 (0) | 10 (2) | 0.487 |
| Smoker (%, ever) (n) | 60 (12) | 35 (7) | 0.113 |
| CRP (mg/L) | 5.8 ± 11.4 | 2.0 ± 1.4 | 0.381 |
| Urea (mmol/L) | 24.0 ± 7.4 | 4.7 ± 1.0 | 0.001 |
| Creatinine (µmol/l) | 926.8 ± 261.6 | 82.2 ± 12.2 | 0.001 |
| Hemoglobin (mmol/l) | 7.1 ± 0.5 | 8.8 ± 1.0 | 0.001 |
| Hematocrit (l/l) | 0.3 ± 0.02 | 0.4 ± 0.04 | 0.001 |
| Ca (mmol/l) | 1.6 ± 0.5 | 2.4 ± 0.09 | 0.001 |
| Na (mmol/l) | 139.5 ± 3.1 | 141.4 ± 2.8 | 0.013 |
| K (mmol/l) | 5.2 ± 0.6 | 4.0 ± 0.5 | 0.001 |
| Leucocytes (G/µl) | 6.6 ± 2.3 | 6.7 ± 2.1 | 0.893 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, C.; Wildgrube, S.; Fiedler, R.; Seibert, E.; Kneser, L.; Fick, S.; Schäfer, C.; Markau, S.; Trojanowicz, B.; Girndt, M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins 2020, 12, 675. https://doi.org/10.3390/toxins12110675
Ulrich C, Wildgrube S, Fiedler R, Seibert E, Kneser L, Fick S, Schäfer C, Markau S, Trojanowicz B, Girndt M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins. 2020; 12(11):675. https://doi.org/10.3390/toxins12110675
Chicago/Turabian StyleUlrich, Christof, Susann Wildgrube, Roman Fiedler, Eric Seibert, Leonie Kneser, Sylvia Fick, Christoph Schäfer, Silke Markau, Bogusz Trojanowicz, and Matthias Girndt. 2020. "NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function" Toxins 12, no. 11: 675. https://doi.org/10.3390/toxins12110675





