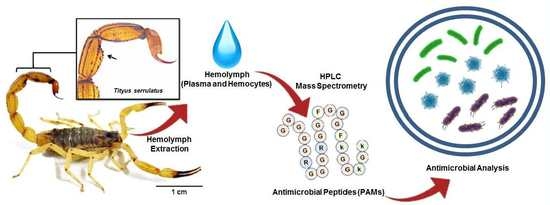
Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fractionation of Peptides from the Hemocytes
2.2. Bioassay
2.2.1. Antimicrobial Activity, Minimal Inhibitory Concentration (MIC)
2.2.2. Hemolytic Activity
2.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel (SDS-PAGE)
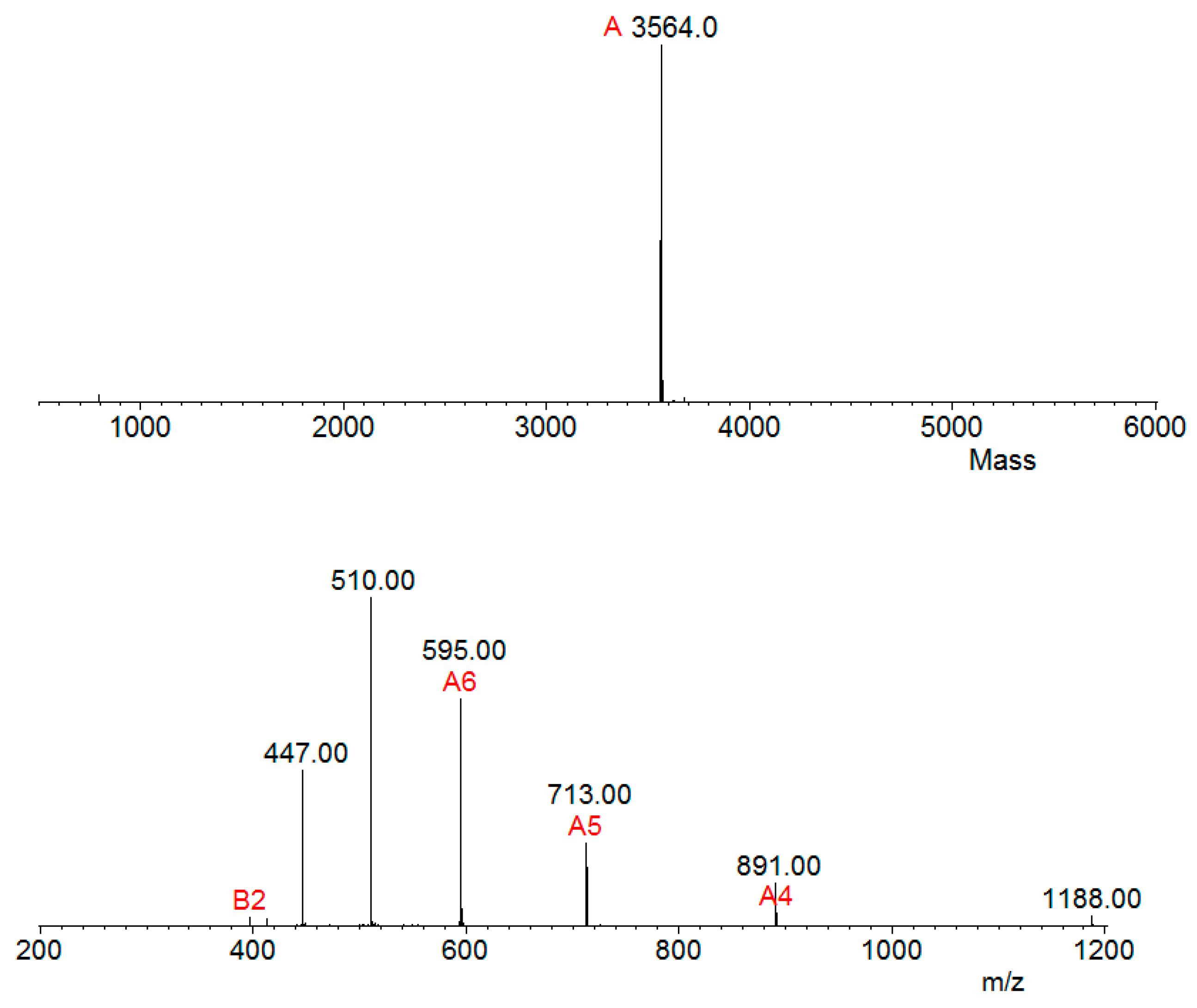
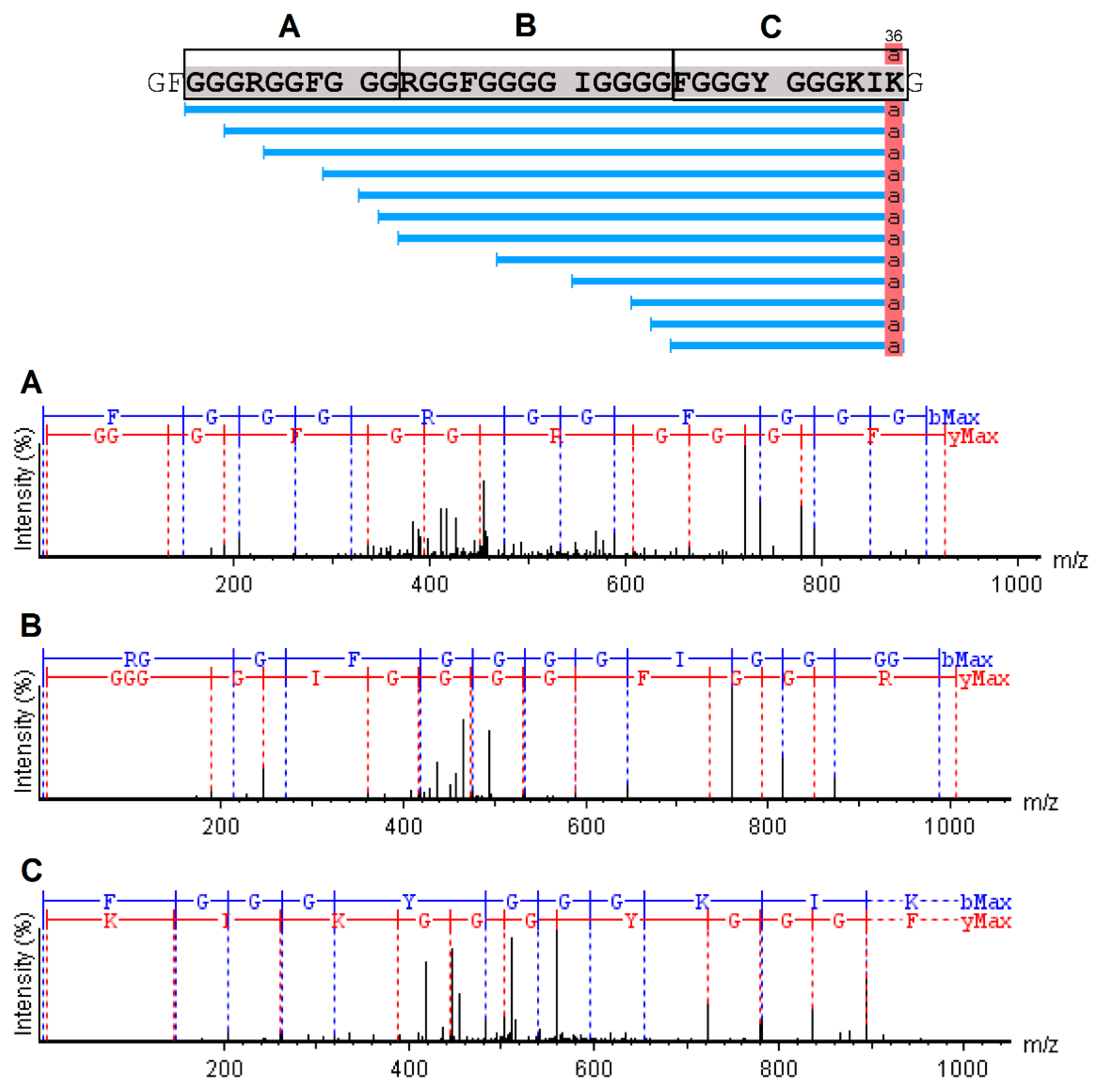
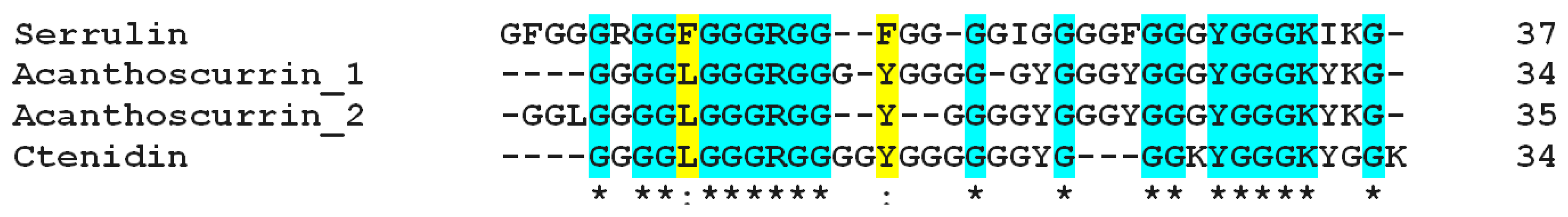
2.4. Mass Spectrometry and Bioinformatics Analyses
3. Conclusions
4. Material and Methods
4.1. Animals and Hemolymph Extraction
4.2. Fractionation of Antimicrobial Peptides
4.3. Bioassays
4.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel (SDS-PAGE)
4.5. Enzymatic “in Gel” Digestion
4.6. Mass Spectrometry and Bioinformatics Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cologna, C.T.; Marcussi, S.; Giglio, J.R.; Soares, A.M.; Arantes, E.C. Tityus serrulatus scorpion venom and toxins: An overview. Protein Pept. Lett. 2009, 16, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Cella, D.M. Karyotype conservation in 2 populations of the parthenogenetic scorpion Tityus serrulatus (Buthidae): rDNA and its associated heterochromatin are concentrated on only one chromosome. J. Hered. 2010, 101, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Merino, E.; Corona, M.; Bolivar, F.; Becerril, B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 2000, 82, 861–868. [Google Scholar] [CrossRef]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Mahadevappa, R.; Ma, R.; Kwok, H.F. Venom Peptides: Improving Specificity in Cancer Therapy. Trends Cancer 2017, 3, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, A.H.; Vellutini, B.C.; Lorenzini, D.M.; da Silva Junior, P.I.; Mortara, R.A.; da Silva, J.M.C.; Daffre, S. The roles of hemocytes in the immunity of the spider Acanthoscurria gomesiana. Dev. Comp. Immunol. 2008, 32, 716–725. [Google Scholar] [CrossRef]
- Nentwing, L.K.; Nentwing, W. The Immune System of Spider. In Spider Ecophysiology; Nentwing, W., Ed.; Springer: Bern, Switzerland, 2013; 82p. [Google Scholar]
- Froy, O.; Gurevitz, M. Arthropod and mollusk defensins-evolution by exon-shuffling. Trends Genet. 2003, 19, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Kuhn-Nentwig, L.; Largiadèr, C.R.; Nentwig, W. Expression of defensins in non-infected araneomorph spiders. Cell. Mol. Life Sci. 2010, 67, 2643–2651. [Google Scholar] [CrossRef] [Green Version]
- Cociancich, S.; Goyffon, M.; Bontems, F.; Bulet, P.; Bouet, F.; Menez, A.; Hoffmann, J. Purification and characterization of a scorpion defensin, a 4kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem. Biophys. Res. Commun. 1993, 194, 17–22. [Google Scholar] [CrossRef]
- Ehret-Sabatier, L.; Loew, D.; Goyffon, M.; Fehlbaum, P.; Hoffmann, J.A.; van Dorsselaer, A.; Bulet, P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef]
- De La Vega, R.R.; Garcia, B.I.; D’Ambrosio, C.; Diego-Garcia, E.; Scaloni, A.; Possani, L.D. Antimicrobial peptide induction in the haemolymph of the Mexican scorpion Centruroides limpidus limpidus in response to septic injury. Cell. Mol. Life Sci. 2004, 61, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.I., Jr.; Daffre, S.; Bulet, P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J. Biol. Chem. 2000, 275, 33464–33470. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, D.M.; da Silva, P.I., Jr.; Fogaça, A.C.; Bulet, P.; Daffre, S. Acanthoscurrin: A novel glycine-rich antimicrobial peptide constitutively expressed in the hemocytes of the spider Acanthoscurria gomesiana. Dev. Comp. Immunol. 2003, 7, 781–791. [Google Scholar] [CrossRef]
- Baumann, T.; Kämpfer, U.; Schürch, S.; Schaller, J.; Largiadèr, C.; Nentwig, W.; Kuhn-Nentwig, L. Ctenidins: Antimicrobial glycine-rich peptides from the hemocytes of the spider Cupiennius salei. Cell. Mol. Life Sci. 2010, 67, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial Peptides: Primeval molecules or future drogs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef] [PubMed]
- Riciluca, K.C.; Sayegh, R.S.; Melo, R.L.; Silva, P.I., Jr. Rondonin an antifungal peptide from spider (Acanthoscurria rondoniae) haemolymph. Results Immunol. 2012, 2, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, E.; da Silva, P.I., Jr. Lacrain: The first antimicrobial peptide from the body extract of the Brazilian centipede Scolopendra viridicornis. Int. J. Antimicrob. Agents 2016, 48, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.C.L.; Miranda, A.; da Silva, P.I., Jr. Human Antimicrobial Peptide Isolated from Triatoma infestans Haemolymph, Trypanosoma cruzi-Transmitting Vector. Front. Cell. Infect. Microbiol. 2018, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Ikonomopoulou, M.P.; Fernandez-Rojo, M.A.; Pineda, S.S.; Cabezas-Sainz, P.; Winnen, B.; Morales, R.A.V.; Brust, A.; Sánchez, L.; Alewood, P.F.; Ramm, G.A.; et al. Gomesin inhibits melanoma growth by manipulating key signaling cascades that control cell death and proliferation. Sci. Rep. 2018, 8, 11519. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Dimarcq, J.L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Van Dorsselaer, A.; Hoffmann, J.A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J. Biol. Chem. 1993, 268, 14893–14897. [Google Scholar]
- Fuzita, F.J.; Pinkse, M.W.; Patane, J.S.; Juliano, M.A.; Verhaert, P.D.; Lopes, A.R. Biochemical, transcriptomic and proteomic analyses of digestion in the scorpion Tityus serrulatus: Insights into function and evolution of digestion in an ancient arthropod. PLoS ONE 2015, 10, e0123841. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, U.C.; Nishiyama, M.Y., Jr.; Dos Santos, M.B.V.; Santos-da-Silva, A.P.; Chalkidis, H.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, V.A.C.; Junqueira-de-Azevedo, I.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, S. The defensin gene family expansion in the tick Ixodes scapularis. Dev. Comp. Immunol. 2011, 35, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Isogai, E.; Hiramatsu, K.; Sasaki, T. Activity of tick antimicrobial peptide from Ixodes persulcatus (persulcatusin) against cell membranes of drug-resistant Staphylococcus aureus. J. Antibiot. 2017, 70, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Pagel Van Zee, J.; Geraci, N.S.; Guerrero, F.D.; Wikel, S.K.; Stuart, J.J.; Nene, V.M.; Hill, C.A. Tick genomics: The Ixodes genome project and beyond. Int. J. Parasitol. 2007, 37, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Herbinière, J.; Braquart-Varnier, C.; Grève, P.; Strub, J.M.; Frère, J.; Van Dorsselaer, A.; Martin, G. Armadillidin: A novel glycine-rich antibacterial peptide directed against gram-positive bacteria in the woodlouse Armadillidium vulgare (terrestrial isopod, Crustacean). Dev. Comp. Immunol. 2005, 29, 489–499. [Google Scholar] [CrossRef]
- Destoumieux, D.; Bulet, P.; Loew, D.; Van Dorsselaer, A.; Rodriguez, J.; Bachère, E. Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J. Biol. Chem. 1997, 272, 28398–28406. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus): Isolation and chemical structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; John, M.W., Ed.; Humans Press Inc.: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Shrestha, A.; Duwadi, D.; Jukosky, J.; Fiering, S.N. Cecropin-like antimicrobial peptide protects mice from lethal E.coli infection. PLoS ONE 2019, 14, e0220344. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, M.S.; Kim, S.C. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochem. Biophys. Res. Commun. 1996, 218, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, H.; Yu, P.L. Mechanisms of action of ostrich beta-defensins against Escherichia coli. FEMS Microbiol. Lett. 2007, 270, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins:antibacterial peptides from honeybees. EMBO J. 1989, 8, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Söderhäll, K.; Smith, V.J. Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 1983, 7, 229–239. [Google Scholar] [CrossRef]
- Hao, G.; Shi, Y.H.; Tang, Y.L.; Le, G.W. The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptides 2009, 30, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.L.; Sherman, N.E.; Kinter, M.T.; Goldberg, J.B. Comparison of proteins expressed by Pseudomonas aeruginosa strains representing initial and chronic isolates from a cystic fibrosis patient: An analysis by 2-D gel electrophoresis and capillary column liquid chromatography-tandem mass spectrometry. Microbiology 2000, 146, 2495–2508. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Zhang, Z.; Marshall, A.G. A Universal Algorithm for Fast and Automated Charge State Deconvolution of Electrospray Mass-to_charge Ratio Spectra. J. Am. Soc. Mass Spectrom. 1998, 9, 225–233. [Google Scholar] [CrossRef]
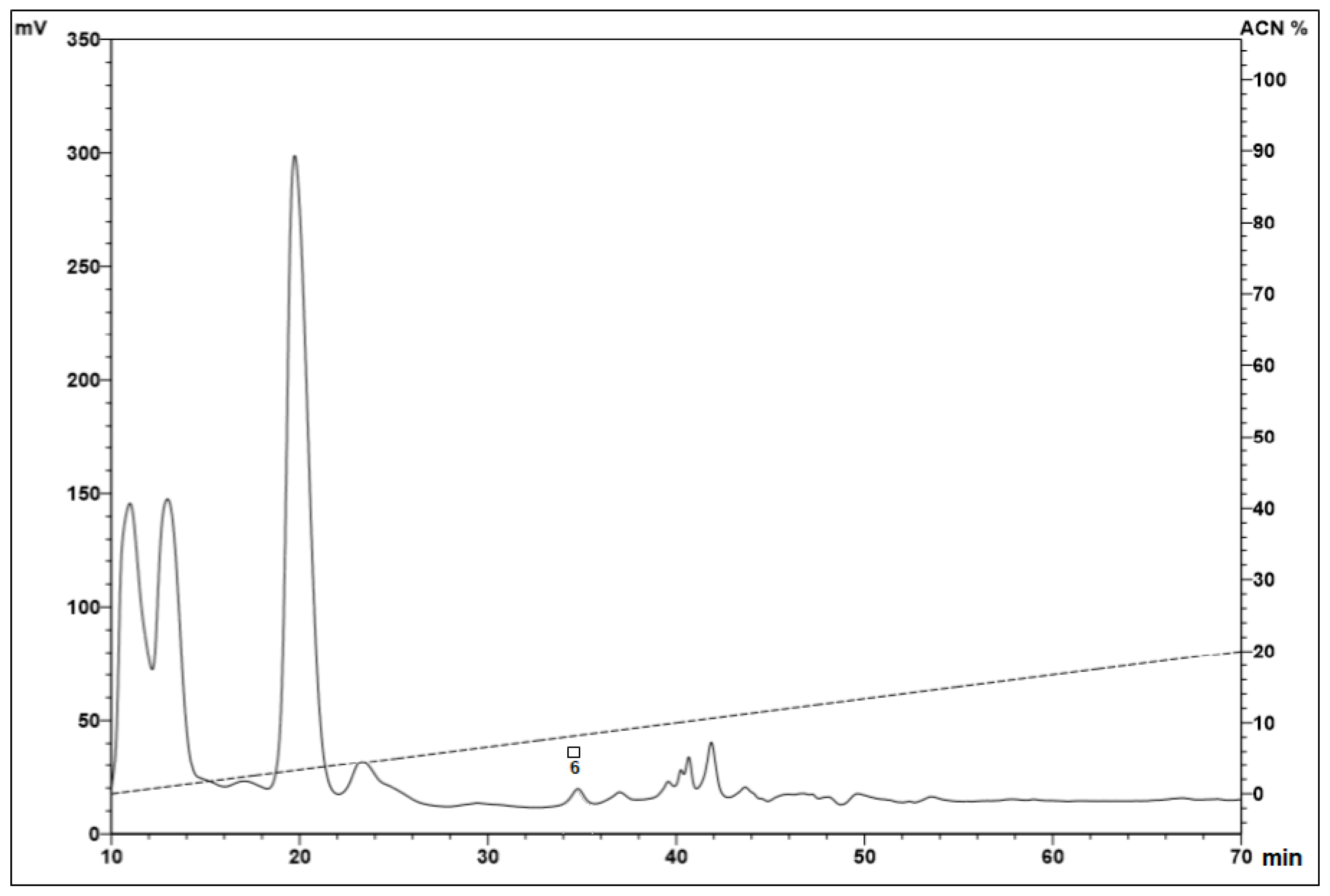
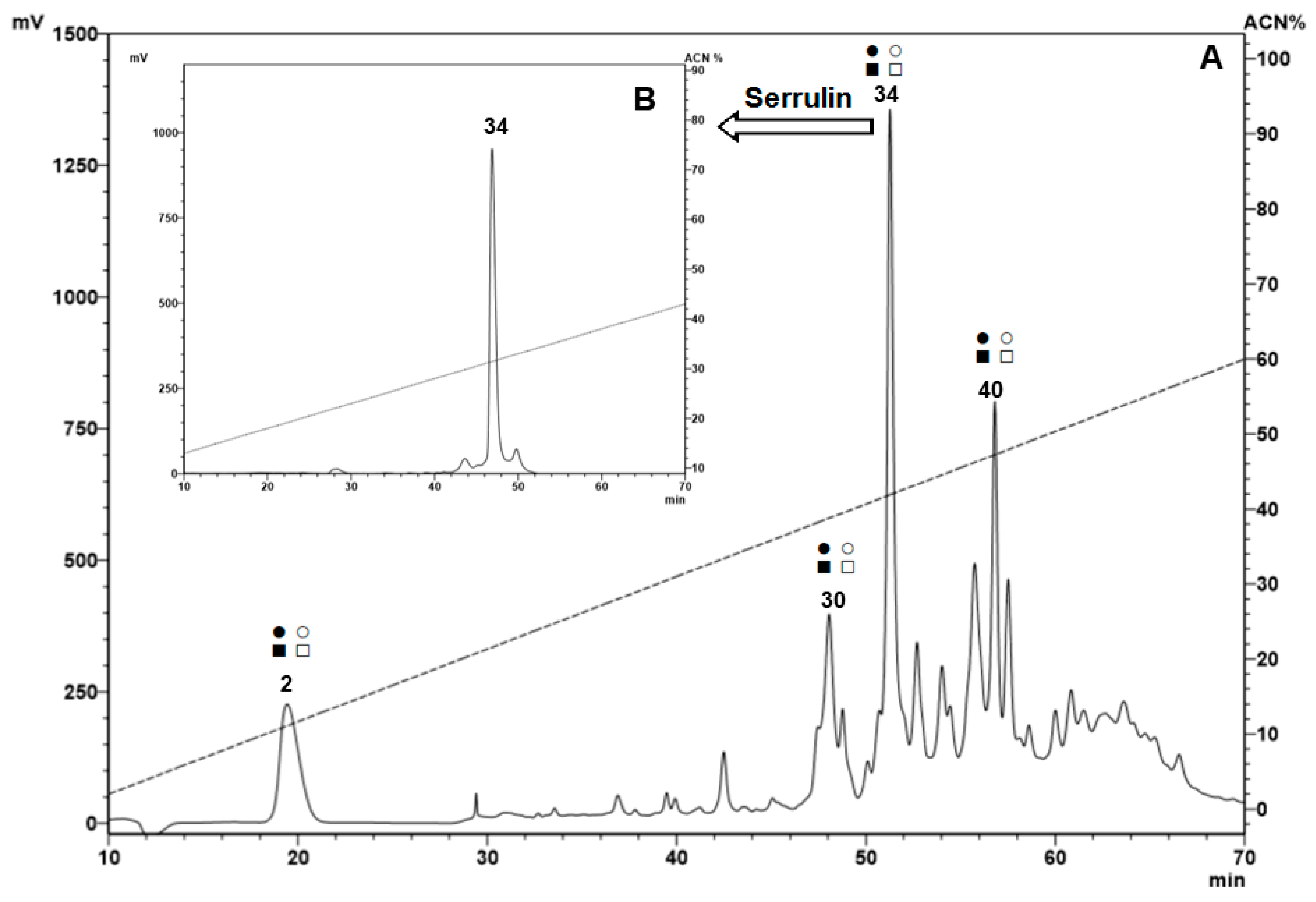
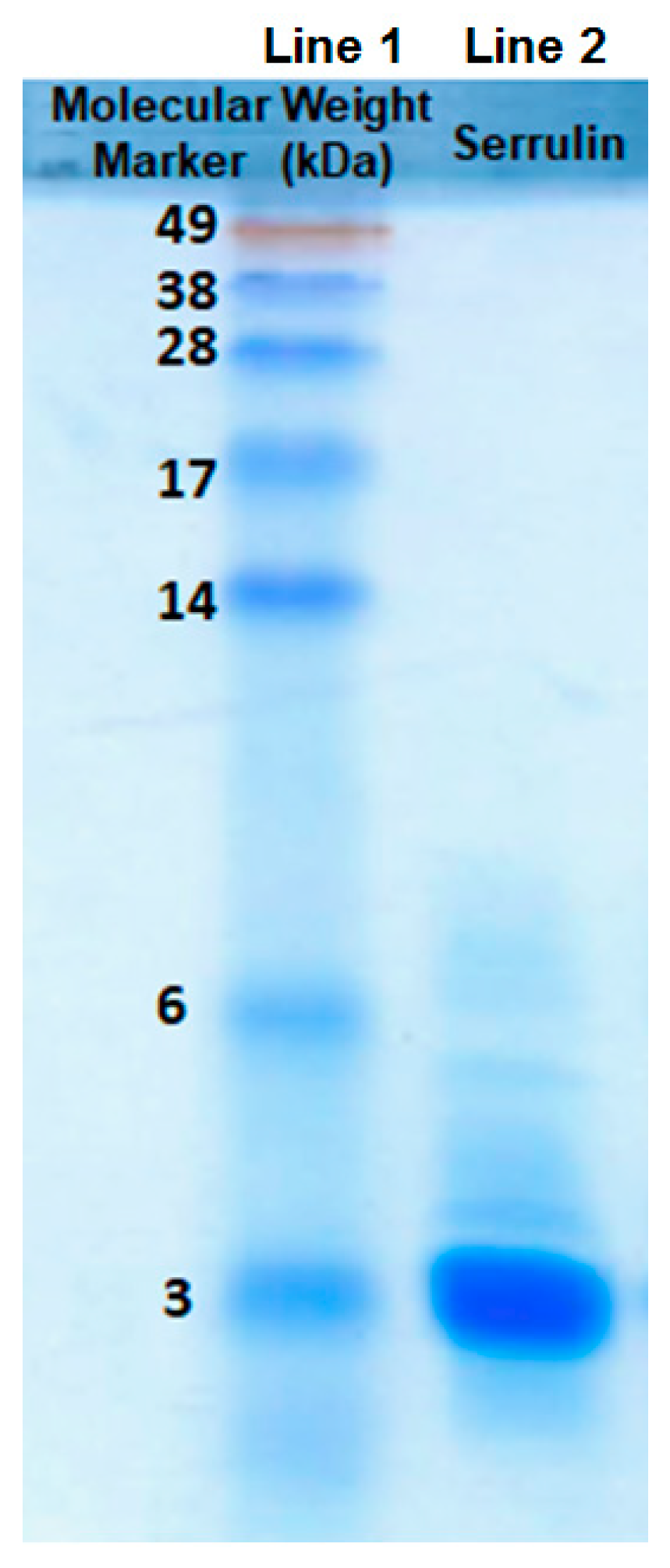
| Microorganism | |||||
|---|---|---|---|---|---|
| Hemocytes | Fractions | Escherichia coli SBS363 | Micrococcus luteus A270 | Candida albicans MDM8 | Aspergillus niger |
| 5% | 6 | – | – | – | + |
| 2 | + | + | + | + | |
| 30 | + | + | + | + | |
| 40% | 34 | + | + | + | + |
| 40 | + | + | + | + | |
| Strain | MIC (µg/mL) | µM |
|---|---|---|
| Gram-positive bacteria | ||
| Micrococcus luteus A270 | 1.87–3.75 | (0.5–1) |
| Gram-negative bacteria | ||
| Escherichia coli SBS 363 | 30–60 | (9–16) |
| Pseudomonas aeruginosa ATCC 27853 | 0.05–0.1 | (0.01–0.3) |
| Fungi | ||
| Aspergillus niger | 12–24 | (3–6) |
| Yeast | ||
| Candida albicans MDM8 | 6–12 | (1.5–3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus Oliveira, T.; Oliveira, U.C.d.; da Silva Junior, P.I. Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion. Toxins 2019, 11, 517. https://doi.org/10.3390/toxins11090517
de Jesus Oliveira T, Oliveira UCd, da Silva Junior PI. Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion. Toxins. 2019; 11(9):517. https://doi.org/10.3390/toxins11090517
Chicago/Turabian Stylede Jesus Oliveira, Thiago, Ursula Castro de Oliveira, and Pedro Ismael da Silva Junior. 2019. "Serrulin: A Glycine-Rich Bioactive Peptide from the Hemolymph of the Yellow Tityus serrulatus Scorpion" Toxins 11, no. 9: 517. https://doi.org/10.3390/toxins11090517