Botulinum Toxin for the Treatment of Hand Tremor
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Description
5.2. Data Collection
5.3. Selection Criteria
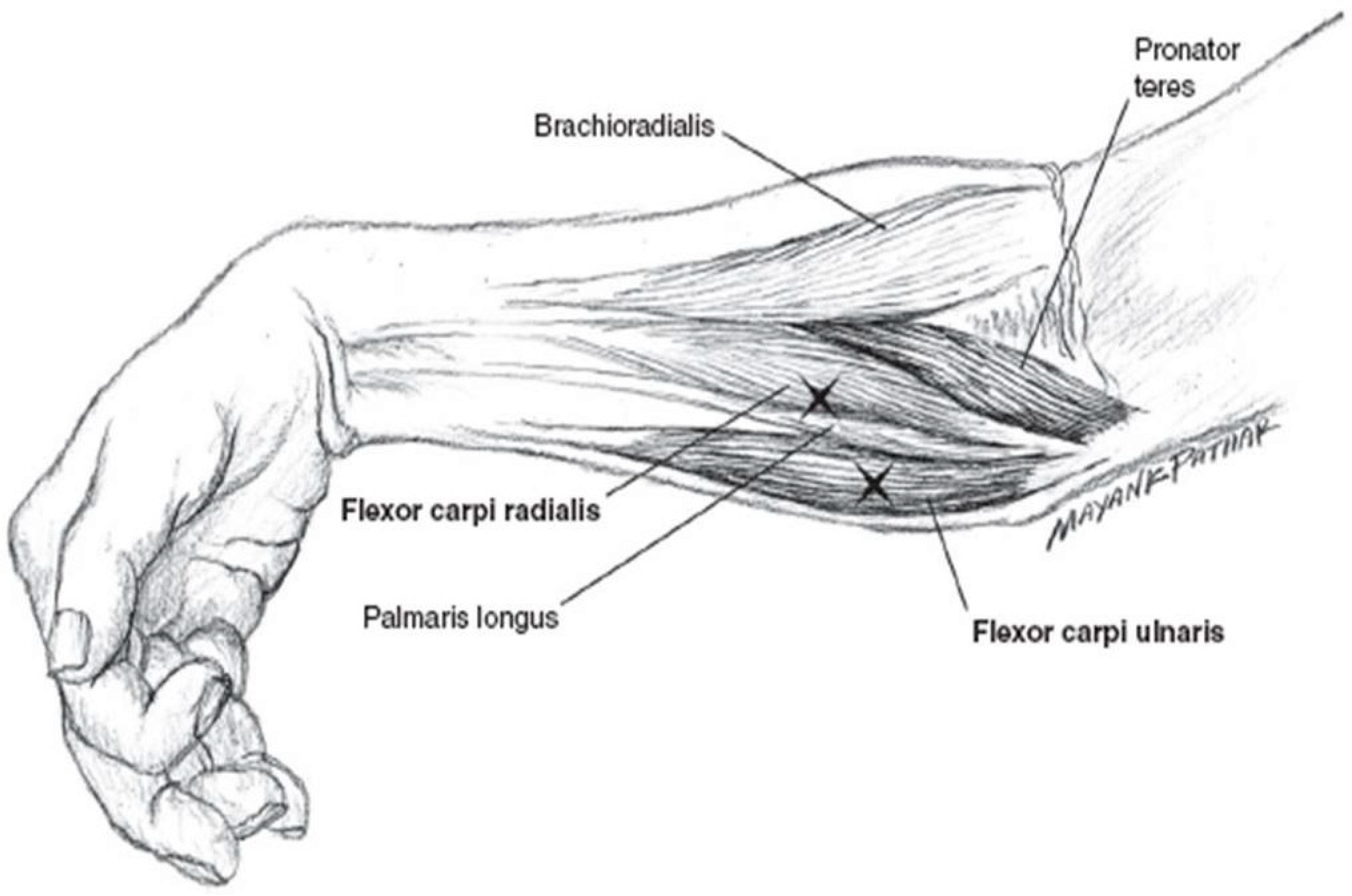
5.4. Botulinum Toxin Injection
5.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Machado, D.G. Tremor-related quality of life: A comparison of essential tremor vs. Parkinson’s disease patients. Park. Relat. Disord. 2015, 21, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Ferreira, J.J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010, 25, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Thenganatt, M.A.; Jankovic, J. The relationship between essential tremor and Parkinson’s disease. Park. Relat. Disord. 2016, 22, S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, M.F.; Zach, H.; Bloem, B.R.; Hallett, M.; Helmich, R.C. The nature of postural tremor in Parkinson disease. Neurology 2018, 90, e1095–e1103. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. How Do I Examine for Re-Emergent Tremor? Mov. Disord. Clin. Pract. 2016, 3, 216–217. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Lang, A.E. The treatment of dystonic tremor: A systematic review. J. Neurol. Neurosurg. Psychiatry 2014, 85, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Sarma, N. Tremor in dystonia. Park. Relat. Disord. 2016, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.C.; Vingerhoets, F.J.G. Tremor revisited: Treatment of PD tremor. Park. Relat. Disord. 2012, 18, 93–95. [Google Scholar] [CrossRef]
- Schneider, S.A.; Deuschl, G. The treatment of tremor. Neurotherapeutics 2014, 11, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, C.; Huckhagel, T.; Engel, K.; Gulberti, A.; Hidding, U.; Poetter-Nerger, M.; Goerendt, I.; Ludewig, P.; Braass, H.; Choe, C.U.; et al. Adverse events in deep brain stimulation: A retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS ONE 2017, 12, e0178984. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Jankovic, J. Movement disorders induced by deep brain stimulation. Park. Relat. Disord. 2016, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schreglmann, S.R.; Krauss, J.K.; Chang, J.W.; Martin, E.; Werner, B.; Bauer, R.; Hägele-Link, S.; Bhatia, K.P.; Kägi, G. Functional lesional neurosurgery for tremor: Back to the future? J. Neurol. Neurosurg. Psychiatry 2018, 89, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J.; Shih, L.; Cozzens, J.W. Surgical treatments for essential tremor. Expert Rev. Neurother. 2018, 18, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Patra, D.; Nanda, A. A meta-analysis of outcomes and complications of magnetic resonance-guided focused ultrasound in the treatment of essential tremor. Neurosurg. Focus 2018, 44. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Castaneda, J.; Jankovic, J.; Comella, C.; Dashtipour, K.; Fernandez, H.H.; Mari, Z. Diffusion, spread, and migration of botulinum toxin. Mov. Disord. 2013, 28, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Castaneda, J.; Jankovic, J. Long-term efficacy, safety, and side effect profile of botulinum toxin in dystonia: A 20-year follow-up. Toxicon 2014, 90, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Rahimi, F.; Lee, J.; Jog, M. Functional ability improved in essential tremor by incobotulinumtoxinA injections using kinematically determined biomechanical patterns—A new future. PLoS ONE 2016, 11, e0153739. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Samotus, O.; Lee, J.; Jog, M. Effective management of upper limb parkinsonian tremor by incobotulinumtoxinA injections using sensor-based biomechanical patterns. Tremor Other Hyperkinet. Mov. 2015, 5. [Google Scholar] [CrossRef]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum toxin in Parkinson disease tremor: A randomized, double-blind, placebo-controlled study with a customized injection approach. Mayo Clin. Proc. 2017, 92, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Jog, M.; Lee, J.; Althaus, M.; Scheschonka, A.; Dersch, H.; Simpson, D.M.; ET Study Team. Efficacy and safety of incobotulinumtoxinA (inco/A) for essential tremor using kinematics-guided clinical decision support: A randomized, double-blind, placebo-controlled trial. Mov. Disord. 2017, 32 (suppl. 2). Available online: http://www.mdsabstracts.org/abstract/efficacy-and-safety-of-incobotulinumtoxina-incoa-for-essential-tremor-using-kinematics-guided-clinical-decision-support-a-randomized-double-blind-placebo-controlled-trial/ (accessed on 18 July 2018).
- Kim, S.D.; Yiannikas, C.; Mahant, N.; Vucic, S.; Fung, V.S. Treatment of proximal upper limb tremor with botulinum toxin therapy. Mov. Disord. 2014, 29, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum toxin in essential hand tremor—A randomized double-blind placebo-controlled study with customized injection approach. Park. Relat. Disord. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Schwartz, K.S. Botulinum toxin treatment of tremors. Neurology 1991, 41, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Schwartz, K.; Clemence, W.; Aswad, A.; Mordaunt, J. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov. Disord. 1996, 11, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Lyons, K.E.; Doucette, J.; Adler, C.H.; Caviness, J.N.; Comella, C.L.; Dubinsky, R.M.; Friedman, J.H.; Manyam, B.V.; Matsumoto, J.Y.; et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology 2001, 56, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. The use of botulinum toxin in tic disorders and essential hand and head tremor. In Manual of Botulinum Toxin Therapy, 2nd ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 160–167. [Google Scholar]
- Rahimi, F.; Bee, C.; Debicki, D.; Roberts, A.C.; Bapat, P.; Jog, M. Effectiveness of BoNT A in Parkinson’s disease upper limb tremor management. Can. J. Neurol. Sci. 2013, 40, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Kumar, N.; Rizek, P.; Jog, M. Botulinum toxin type A injections as monotherapy for upper limb essential tremor using kinematics. Can. J. Neurol. Sci. 2018, 45, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Lee, J.; Jog, M. Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections. PLoS ONE 2017, 12, e0178670. [Google Scholar] [CrossRef] [PubMed]
- Zakin, E.; Simpson, D. Botulinum toxin in management of limb tremor. Toxins 2017, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Karp, B.; Alter, K. Muscle selection for focal limb dystonia. Toxins 2017, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Pacchetti, C.; Mancini, F.; Bulgheroni, M.; Zangaglia, R.; Cristina, S.; Sandrini, G.; Nappi, G. Botulinum toxin treatment for functional disability induced by essential tremor. Neurol. Sci. 2000, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sitburana, O.; Wu, L.J.; Sheffield, J.K.; Davidson, A.; Jankovic, J. Motor overflow and mirror dystonia. Park. Relat. Disord. 2009, 15, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xue, F.; Chang, W.; Lian, Y.; Zheng, Y.; Xie, N.; Zhang, L.; Chen, C. Botulinum toxin type A with or without needle electromyographic guidance in patients with cervical dystonia. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Needle EMG guidance for injection of botulinum toxin. Needle EMG guidance is rarely required. Muscle Nerve 2001, 24, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.K.; Jankovic, J. Botulinum toxin in the treatment of tremors, dystonias, sialorrhea and other symptoms associated with Parkinson’s disease. Expert Rev. Neurother. 2007, 7, 637–647. [Google Scholar] [CrossRef] [PubMed]

| Mean | SD | Range | |
|---|---|---|---|
| Age at first injection (years) | 64.8 | 12.8 | 18–93 |
| Tremor duration at first injection (years) | 23.2 | 17.5 | 0.5–75 |
| Follow-up period (months) | 29.6 | 25.1 | 3–88 |
| Number of onaBoNT-A sessions | 7.7 | 6.3 | 2–31 |
| OnaBoNT-A units per session † | 71.8 | 37.3 | 22.5–225 |
| OnaBoNT-A units † (per treatment indication) | |||
| ET (n = 53) | 71.3 | 36.7 | 22.5–225 |
| PD (n = 6) | 47.9 | 11.5 | 37.5–67.5 |
| Dystonia (n = 31) | 77.3 * | 41.0 | 27.5–187.5 |
| COT (n = 1) | 70 | - | - |
| Treatment Indication (Muscles Injected/Limbs Injected) | |||||
|---|---|---|---|---|---|
| ET | PD | Dystonia | COT | Total | |
| Deltoid | 0/99 | 0/6 | 1/37 | 1/1 | 2/143 |
| Biceps | 10/99 | 0/6 | 3/37 | 1/1 | 14/143 |
| Triceps | 1/99 | 0/6 | 0/37 | 0/1 | 1/143 |
| Pronator teres | 4/99 | 0/6 | 5/37 | 0/1 | 9/143 |
| FCU | 94/99 | 6/6 | 34/37 | 1/1 | 135/143 |
| FCR | 92/99 | 6/6 | 26/37 | 1/1 | 125/143 |
| FDS | 3/99 | 0/6 | 4/37 | 0/1 | 7/143 |
| ADM | 0/99 | 0/6 | 1/37 | 0/1 | 1/143 |
| APB | 1/99 | 0/6 | 11/37 | 0/1 | 12/143 |
| ED | 0/99 | 0/6 | 1/37 | 0/1 | 1/143 |
| EPB | 0/99 | 0/6 | 1/37 | 0/1 | 1/143 |
| UNS extensor | 0/99 | 0/6 | 1/37 | 0/1 | 1/143 |
| First Injection (n) | Last Injection (n) | p * | |
|---|---|---|---|
| Mean onaBoNT-A units | 65.0 ± 31.2 (91) | 78.6 ± 51.1 (91) | 0.002 |
| Mean global rating | 3.0 ± 1.3 (91) | 3.2 ± 1.2 (91) | 0.06 |
| Mean peak effect | 3.2 ± 1.3 (91) | 3.4 ± 1.1 (91) | 0.18 |
| Moderate or marked benefit (score of 3 or 4) | |||
| Global rating | 74.7% | 80.2% | - |
| Peak effect | 80.2% | 85.7% | - |
| Mean latency of response, days | 4.5 ± 4.3 (81) | 3.8 ± 3.0 (78) | 0.10 |
| Mean total duration of response, weeks † | 12.7 ± 3.6 (46) | 12.8 ± 2.8 (37) | 0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemann, N.; Jankovic, J. Botulinum Toxin for the Treatment of Hand Tremor. Toxins 2018, 10, 299. https://doi.org/10.3390/toxins10070299
Niemann N, Jankovic J. Botulinum Toxin for the Treatment of Hand Tremor. Toxins. 2018; 10(7):299. https://doi.org/10.3390/toxins10070299
Chicago/Turabian StyleNiemann, Nicki, and Joseph Jankovic. 2018. "Botulinum Toxin for the Treatment of Hand Tremor" Toxins 10, no. 7: 299. https://doi.org/10.3390/toxins10070299





