Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model
Abstract
1. Introduction
2. Results
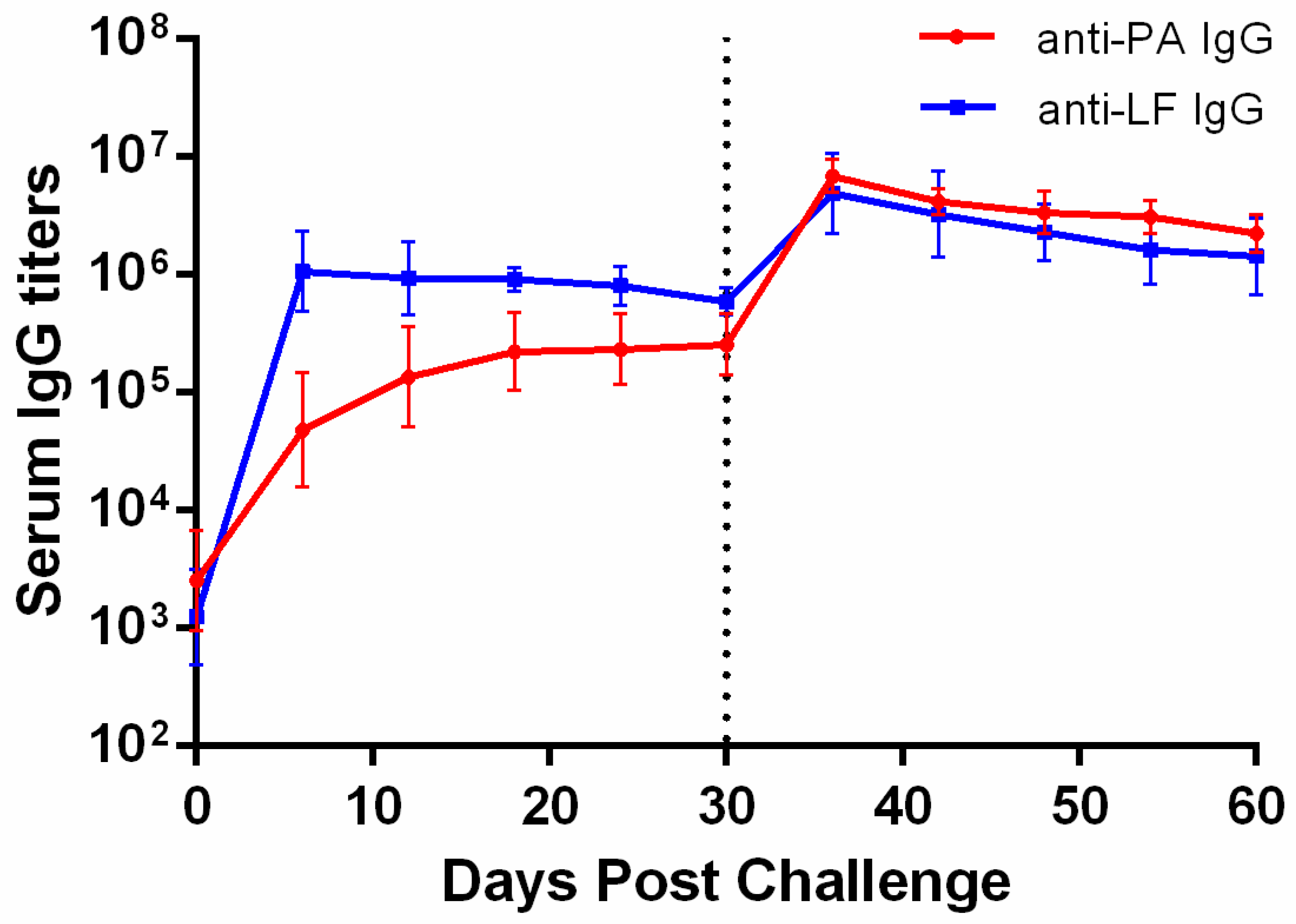
2.1. Establishment of the NZW Rabbit Model for LT Challenge
2.2. 5E11 Pharmacokinetics (PK) in NZW Rabbits
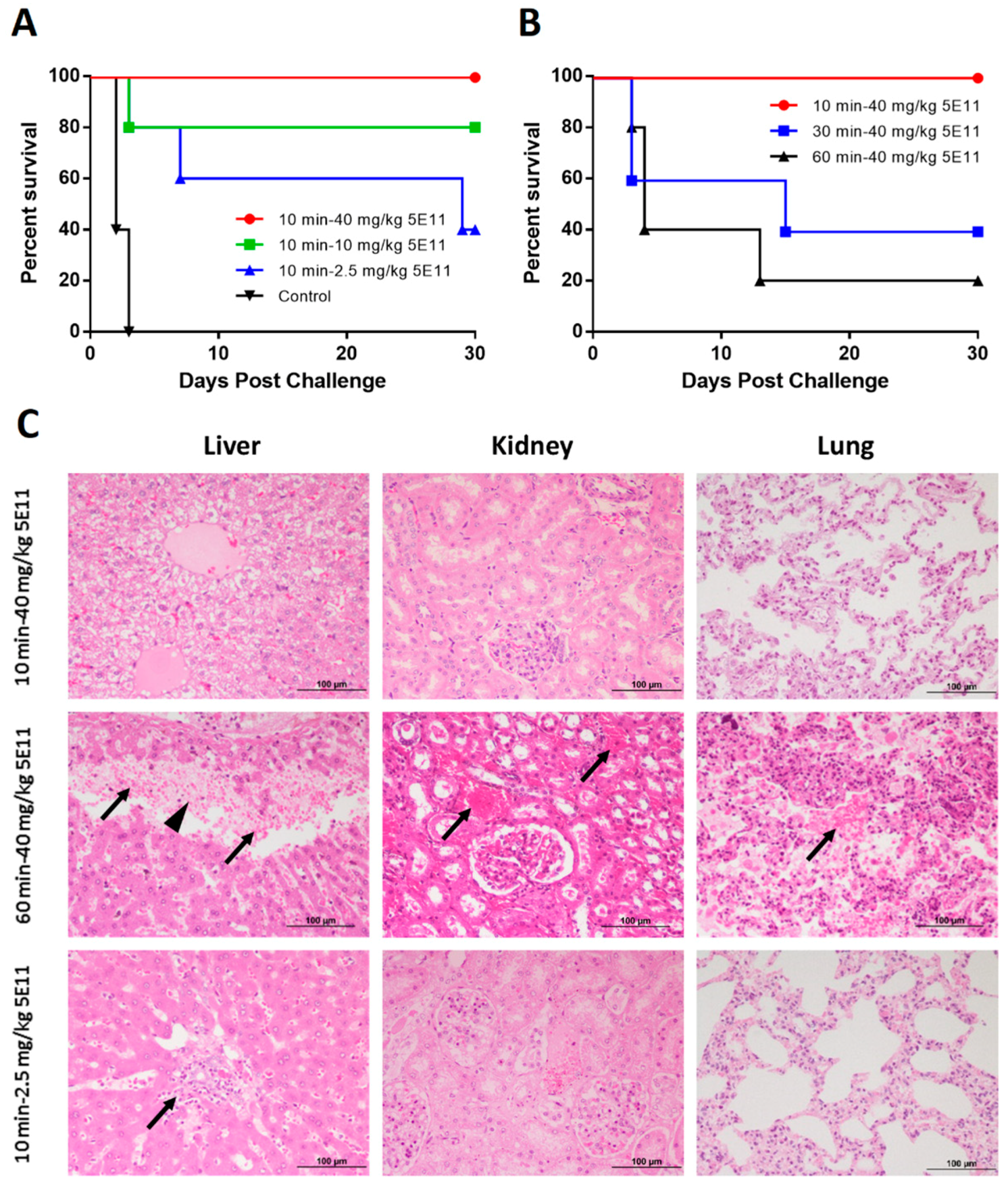
2.3. Prophylactic and Therapeutic Efficacy in NZW Rabbits
3. Discussion
4. Materials and Methods
4.1. Study Designs
4.2. Animals
4.3. LT and 5E11 MAb Preparation
4.4. LT Challenge and Administration
4.5. Clinical Observation
4.6. Cytokine Assay
4.7. Pathology
4.8. Blood Culture and Bacterial Identification
4.9. Pharmacokinetics (PK) Parameters
4.10. Detection of Rabbit Anti-PA and Anti-LF Polyclonal IgG
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hicks, C.W.; Sweeney, D.A.; Cui, X.; Li, Y.; Eichacker, P.Q. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med. 2012, 38, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Candela, T.; Fouet, A. Poly-gamma-glutamate in bacteria. Mol. Microbiol. 2006, 60, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, M.; Heninger, S.; Hutt, J.; Chen, Y.; Lyons, C.R.; Koehler, T.M. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 2005, 24, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Head, B.M.; Rubinstein, E.; Meyers, A.F. Alternative pre-approved and novel therapies for the treatment of anthrax. BMC Infect. Dis 2016, 16, 621. [Google Scholar] [CrossRef] [PubMed]
- Dixon, T.C.; Meselson, M.; Guillemin, J.; Hanna, P.C. Anthrax. N. Engl. J. Med. 1999, 341, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.N.; Rossi Paccani, S.; Quesnel-Hellmann, A.; Baldari, C.T. Anthrax toxins: A weapon to systematically dismantle the host immune defenses. Mol. Asp. Med. 2009, 30, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Leppla, S.H. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol. Asp. Med. 2009, 30, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Moayeri, M.; Purcell, R. Monoclonal antibody therapies against anthrax. Toxins 2011, 3, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Ionin, B.; Hopkins, R.J.; Pleune, B.; Sivko, G.S.; Reid, F.M.; Clement, K.H.; Rudge, T.L., Jr.; Stark, G.V.; Innes, A.; Sari, S.; et al. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin. Vaccine Immunol. CVI 2013, 20, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Athamna, A.; Athamna, M.; Abu-Rashed, N.; Medlej, B.; Bast, D.J.; Rubinstein, E. Selection of Bacillus anthracis isolates resistant to antibiotics. J. Antimicrob. Chemother. 2004, 54, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Price, L.B.; Vogler, A.; Pearson, T.; Busch, J.D.; Schupp, J.M.; Keim, P. In vitro selection and characterization of Bacillus anthracis mutants with high-level resistance to ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax pathogenesis. Annu Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, J.; Li, B.; Zhang, J.; Hou, L.; Song, X.; Yu, T.; Xu, J.; Fu, L.; Zhang, J.; et al. A Monoclonal Antibody That Neutralize Anthrax Protective Antigen (PA) Toxin and Its Application. Patent ZL201210361845.4, 2012. [Google Scholar]
- Zaucha, G.M.; Pitt, L.M.; Estep, J.; Ivins, B.E.; Friedlander, A.M. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 1998, 122, 982–992. [Google Scholar] [PubMed]
- Peterson, J.W.; Comer, J.E.; Noffsinger, D.M.; Wenglikowski, A.; Walberg, K.G.; Chatuev, B.M.; Chopra, A.K.; Stanberry, L.R.; Kang, A.S.; Scholz, W.W. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 2006, 74, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Migone, T.S.; Subramanian, G.M.; Zhong, J.; Healey, L.M.; Corey, A.; Devalaraja, M.; Lo, L.; Ullrich, S.; Zimmerman, J.; Chen, A. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009, 361, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Biron, B.; Beck, K.; Dyer, D.; Mattix, M.; Twenhafel, N.; Nalca, A. Efficacy of ETI-204 monoclonal antibody as an adjunct therapy in a New Zealand white rabbit partial survival model for inhalational anthrax. Antimicrob. Agents Chemother. 2015, 59, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, B.J.; Shadiack, A.M.; Carpenter, S.; Sanford, D.; Henning, L.N.; O’Connor, E.; Gonzales, N.; Mondick, J.; French, J.; Stark, G.V.; et al. Efficacy projection of obiltoxaximab for treatment of inhalational anthrax across a range of disease severity. Antimicrob. Agents Chemother. 2016, 60, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, B.J.; Shadiack, A.M.; Carpenter, S.; Sanford, D.; Henning, L.N.; Gonzales, N.; O’Connor, E.; Casey, L.S.; Serbina, N.V. Obiltoxaximab prevents disseminated Bacillus anthracis infection and improves survival during pre- and postexposure prophylaxis in animal models of inhalational anthrax. Antimicrob. Agents Chemother. 2016, 60, 5796–5805. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ohanjandian, L.; Sun, J.; Cui, X.; Suffredini, D.; Yan, L.; Welsh, J.; Eichacker, P.Q. A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results. PLoS ONE 2017, 12, e0182879. [Google Scholar] [CrossRef] [PubMed]
- Comer, J.E.; Ray, B.D.; Henning, L.N.; Stark, G.V.; Barnewall, R.E.; Mott, J.M.; Meister, G.T. Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand white rabbits as a trigger for treatment. Clin. Vaccine Immunol. CVI 2012, 19, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Malkevich, N.V.; Hopkins, R.J.; Bernton, E.; Meister, G.T.; Vela, E.M.; Atiee, G.; Johnson, V.; Nabors, G.S.; Aimes, R.T.; Ionin, B.; et al. Efficacy and safety of AVP-21D9, an anthrax monoclonal antibody, in animal models and humans. Antimicrob. Agents Chemother. 2014, 58, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Mytle, N.; Hopkins, R.J.; Malkevich, N.V.; Basu, S.; Meister, G.T.; Sanford, D.C.; Comer, J.E.; Van Zandt, K.E.; Al-Ibrahim, M.; Kramer, W.G. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob. Agents Chemother. 2013, 57, 5684. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Sun, C.; Xu, L.X.; Owen, R.J.; Auth, R.D.; Snoy, P.J.; Frucht, D.M. Neutrophil elastase mediates pathogenic effects of anthrax lethal toxin in the murine intestinal tract. J. Immunol. 2010, 185, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Shu, O.; Moayeri, M.; Eckhaus, M.A.; Crown, D.; Millerrandolph, S.; Liu, S.; Akira, S.; Leppla, S.H. Myd88-dependent signaling protects against anthrax lethal toxin-induced impairment of intestinal barrier function. Infect. Immun. 2011, 79, 118–124. [Google Scholar]
- Xie, T.; Auth, R.D.; Frucht, D.M. The effects of anthrax lethal toxin on host barrier function. Toxins 2011, 3, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hui, F.; Tao, X.; Auth, R.D.; Patel, N.; Murray, P.R.; Snoy, P.J.; Frucht, D.M. Anthrax lethal toxin disrupts intestinal barrier function and causes systemic infections with enteric bacteria. PLoS ONE 2012, 7, e33583. [Google Scholar]
- Moayeri, M.; Leysath, C.E.; Tremblay, J.M.; Vrentas, C.; Crown, D.; Leppla, S.H.; Shoemaker, C.B. A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model. J. Biol. Chem. 2015, 290, 6584–6595. [Google Scholar] [CrossRef] [PubMed]
- Corey, A.; Migone, T.S.; Bolmer, S.; Fiscella, M.; Ward, C.; Chen, C.; Meister, G. Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/raxibacumab-treated New Zealand white rabbits. Toxins 2013, 5, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Kobiler, D.; Levy, H.; Pass, A.; Ophir, Y.; Rothschild, N.; Tal, A.; Schlomovitz, J.; Altboum, Z. Antibiotics cure anthrax in animal models. Antimicrob. Agents Chemother. 2011, 55, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Pillai, S.K.; Bower, W.A.; Hendricks, K.A.; Guarnizo, J.T.; Hoyle, J.D.; Gorman, S.E.; Boyer, A.E.; Quinn, C.P.; Meaney-Delman, D. Antitoxin treatment of inhalation anthrax: A systematic review. Health Secur. 2015, 13, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, C.; Guo, Q.; Zhang, J.; Dong, D.; Li, G.; Fu, L.; Xu, J.; Chen, W. Secretory expression and efficient purification of recombinant anthrax toxin lethal factor with full biological activity in E. coli. Protein Expr. Purif. 2013, 89, 56–61. [Google Scholar] [CrossRef] [PubMed]

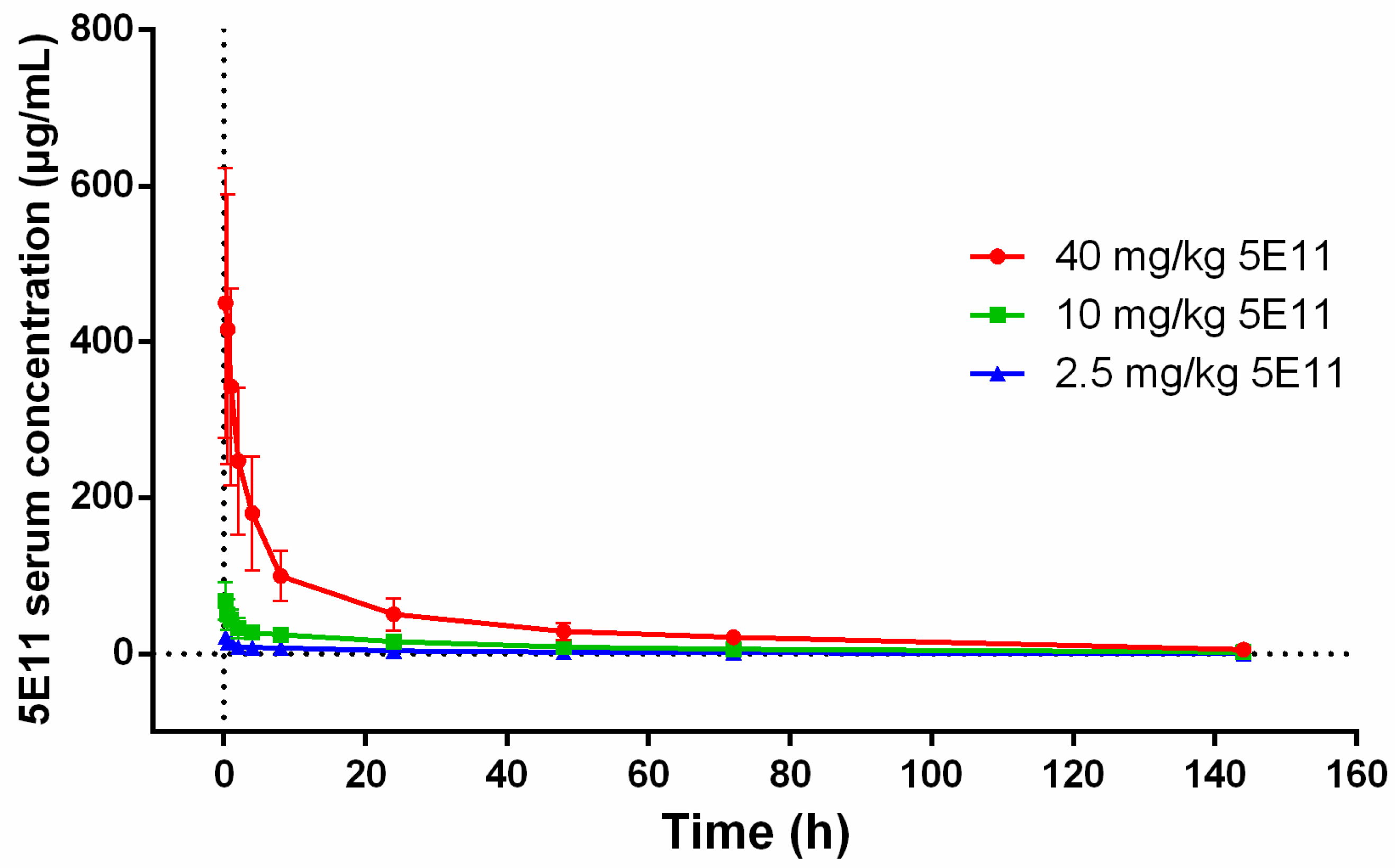
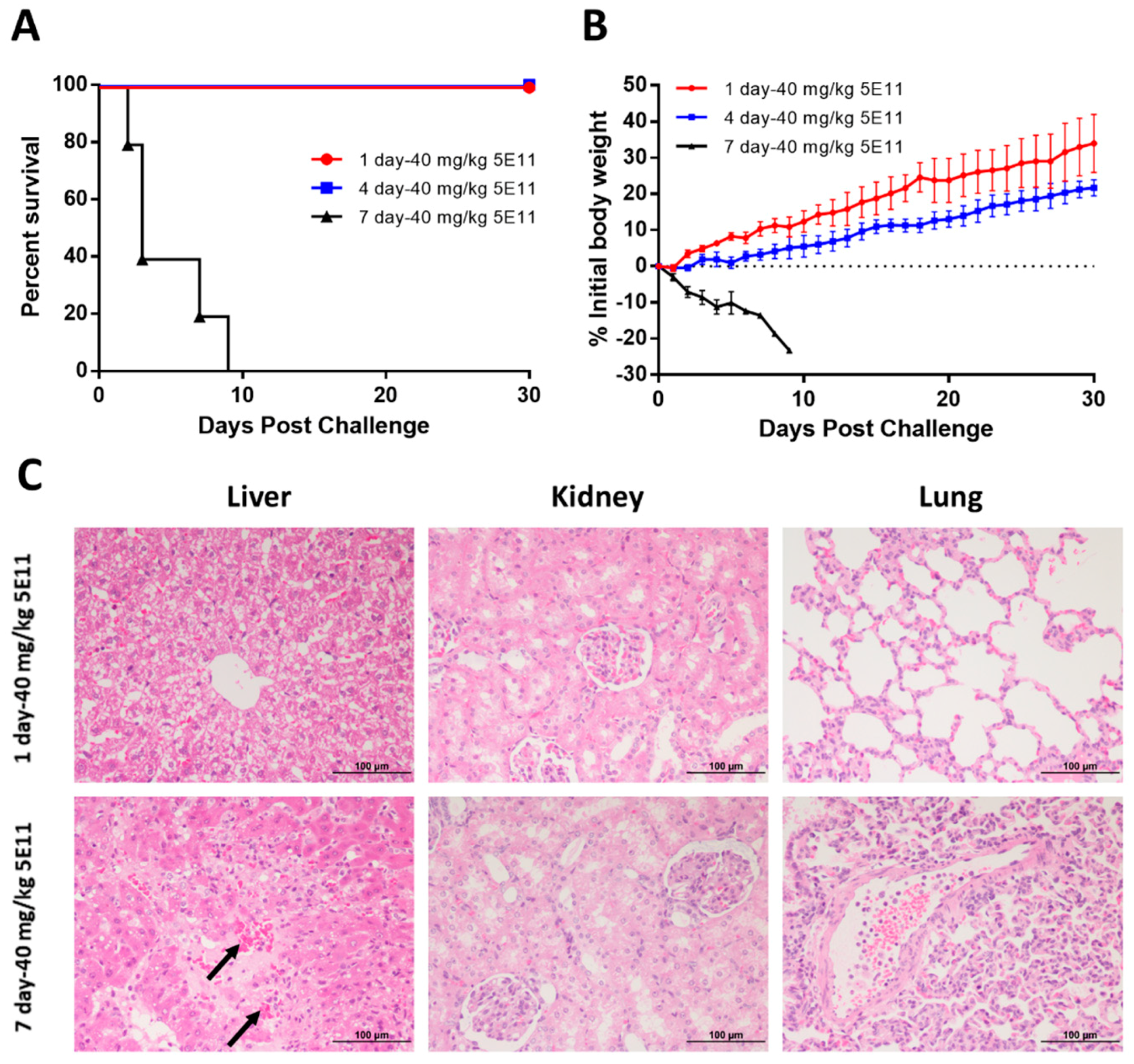
| Parameter a | Results (Mean [SD]) by 5E11 Dose (mg/kg) Group: | ||
|---|---|---|---|
| 40 | 10 | 2.5 | |
| C0 (μg/mL) | 487 (299) | 93 (54) | 34 (21) |
| AUC0–144 h (h·μg/mL) | 5268 (3613) | 1311 (361) | 329 (78) |
| AUC0–∞ (h·μg/mL) | 5537 (3541) | 1375 (351) | 348 (80) |
| t1/2 (h) | 34 (7) | 33 (3) | 40 (4) |
| MRT (h) | 26 (9) | 37 (3) | 33 (1) |
| CL (mL/h/kg) | 11.1 (9.5) | 7.6 (1.7) | 7.5 (1.9) |
| Vz (mL/kg) | 583 (541) | 369 (112) | 436 (158) |
| Study | Study Design | Treatment Regimen and/or Dose | LT-Challenged Dose | No. of Animals |
|---|---|---|---|---|
| LRM | LT-challenged rabbit model; dose-ranging study of LT administered via i.v. in challenged animals | N | 4 mg PA + 2 mg LF | 10 |
| N | 2 mg PA + 1 mg LF | 4 | ||
| N | 1 mg PA + 0.5 mg LF | 4 | ||
| N | PBS | 4 | ||
| Preexposure | Prophylaxis; i.v. dose administered at 1, 4, and 7 d pre-exposure | 40 mg/kg (1 d) | 4 mg PA + 2 mg LF | 5 |
| 40 mg/kg (4 d) | 4 mg PA + 2 mg LF | 5 | ||
| 40 mg/kg (7 d) | 4 mg PA + 2 mg LF | 5 | ||
| Postexposure 1 | Therapy; i.v. dose-ranging study in challenged animals; dose administered at 10 min post-exposure | 40 mg/kg | 4 mg PA + 2 mg LF | 5 |
| 10 mg/kg | 4 mg PA + 2 mg LF | 5 | ||
| 2.5 mg/kg | 4 mg PA + 2 mg LF | 5 | ||
| Control | 4 mg PA + 2 mg LF | 5 | ||
| Postexposure 2 | Therapy; efficacy of 5E11 administered via i.v. at increasing times post-exposure (10–60 min) | 40 mg/kg (10 min) | 4 mg PA + 2 mg LF | 5 |
| 40 mg/kg (30 min) | 4 mg PA + 2 mg LF | 5 | ||
| 40 mg/kg (60 min) | 4 mg PA + 2 mg LF | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, W.; Wen, Z.; Li, B.; Liu, S.; Li, J.; Chen, W. Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model. Toxins 2018, 10, 289. https://doi.org/10.3390/toxins10070289
Zhang D, Liu W, Wen Z, Li B, Liu S, Li J, Chen W. Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model. Toxins. 2018; 10(7):289. https://doi.org/10.3390/toxins10070289
Chicago/Turabian StyleZhang, Duanyang, Weicen Liu, Zhonghua Wen, Bing Li, Shuling Liu, Jianmin Li, and Wei Chen. 2018. "Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model" Toxins 10, no. 7: 289. https://doi.org/10.3390/toxins10070289
APA StyleZhang, D., Liu, W., Wen, Z., Li, B., Liu, S., Li, J., & Chen, W. (2018). Establishment of a New Zealand White Rabbit Model for Lethal Toxin (LT) Challenge and Efficacy of Monoclonal Antibody 5E11 in the LT-Challenged Rabbit Model. Toxins, 10(7), 289. https://doi.org/10.3390/toxins10070289




