High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein
Abstract
:1. Introduction
2. Results
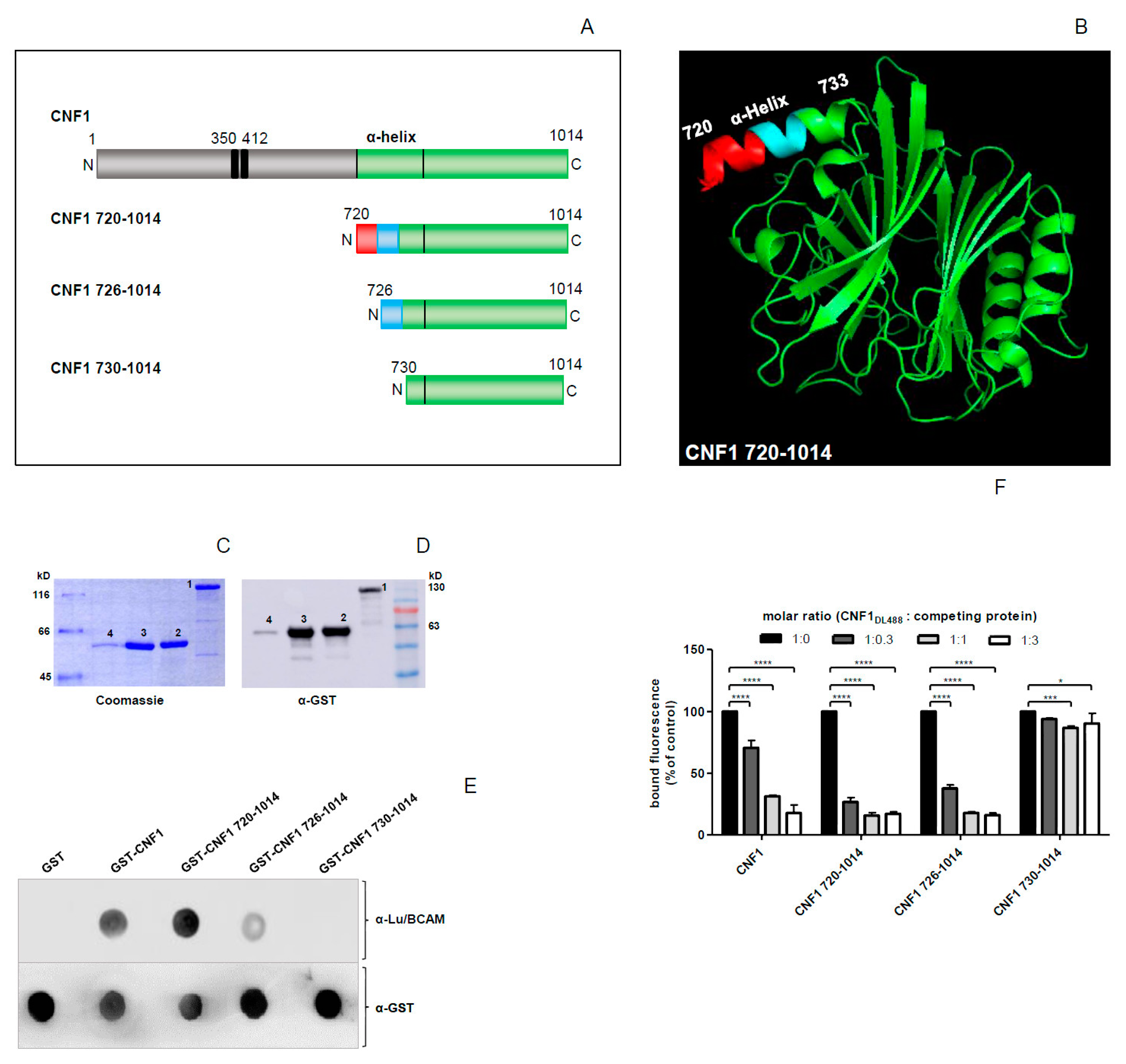
2.1. The C-Terminal Part of CNF1 Interacts with Lu/BCAM
2.2. CNF1 Interacts with the Extracellular Ig-Like Domain 2 of Lu/BCAM
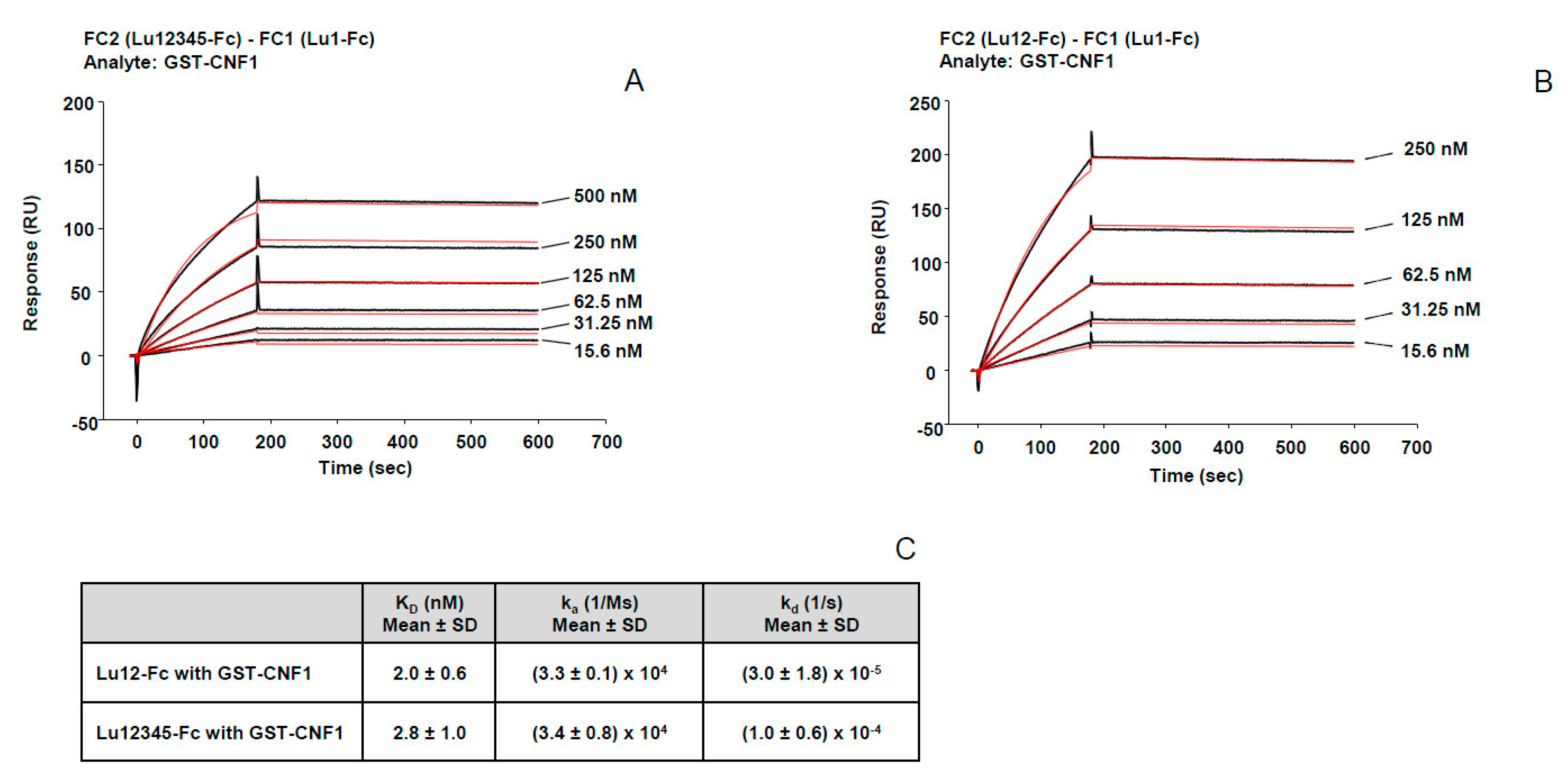
2.3. High Affinity Binding of CNF1 to Lu/BCAM
2.4. Modelling the CNF1–Lu/BCAM Complex
3. Discussion
4. Experimental Procedures
4.1. Cell Culture
4.2. Purification of Recombinant Lu-Fc Proteins
4.3. Cloning, Mutagenesis and Purification of Recombinant CNF Proteins
4.4. SDS-PAGE and Western Blotting
4.5. Dot Blot Binding Studies
4.6. In Vitro Rho Shift
4.7. FACS Analysis
4.8. GST-Based Pulldown Assay
4.9. Surface Plasmon Resonance Measurements
4.10. Purification of the Lu/BCAM-CNF1 Complex by Gel Filtration
4.11. Small-Angle X-ray Scattering (SAXS) Analysis of a Lu/BCAM-CNF1 Complex
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulett, G.C.; Totsika, M.; Schaale, K.; Carey, A.J.; Sweet, M.J.; Schembri, M.A. Uropathogenic Escherichia coli virulence and innate immune responses during urinary tract infection. Curr. Opin. Microbiol. 2013, 16, 100–107. [Google Scholar] [CrossRef] [PubMed]
- DerMardirossian, C.; Bokoch, G.M. Gdis: Central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005, 15, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.; Sehr, P.; Wilm, M.; Selzer, J.; Mann, M.; Aktories, K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 1997, 387, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Flatau, G.; Lemichez, E.; Gauthier, M.; Chardin, P.; Paris, S.; Fiorentini, C.; Boquet, P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 1997, 387, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Lerm, M.; Selzer, J.; Hoffmeyer, A.; Rapp, U.R.; Aktories, K.; Schmidt, G. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1 (CNF1): Activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 1999, 67, 496–503. [Google Scholar] [PubMed]
- Gerhard, R.; Schmidt, G.; Hofmann, F.; Aktories, K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect. Immun. 1998, 66, 5125–5131. [Google Scholar] [PubMed]
- Khan, N.A.; Wang, Y.; Kim, K.J.; Chung, J.W.; Wass, C.A.; Kim, K.S. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 2002, 277, 15607–15612. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, B.; Hoffmann, C.; Aktories, K.; Backert, S.; Schmidt, G. The cytotoxic necrotizing factors from Yersinia pseudotuberculosis and from Escherichia coli bind to different cellular receptors but take the same route to the cytosol. Infect. Immun. 2007, 75, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Knust, Z.; Blumenthal, B.; Aktories, K.; Schmidt, G. Cleavage of Escherichia coli cytotoxic necrotizing factor 1 is required for full biologic activity. Infect. Immun. 2009, 77, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Doye, A.; Boquet, P. Mutation of specific acidic residues of the CNF1 T domain into lysine alters cell membrane translocation of the toxin. Mol. Microbiol. 2001, 41, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Piteau, M.; Papatheodorou, P.; Schwan, C.; Schlosser, A.; Aktories, K.; Schmidt, G. Lu/BCAM adhesion glycoprotein is a receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1). PLoS Pathog. 2014, 10, e1003884. [Google Scholar] [CrossRef]
- Eyler, C.E.; Telen, M.J. The Lutheran glycoprotein: A multifunctional adhesion receptor. Transfusion 2006, 46, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Miner, J.H. Review: Lutheran/B-CAM: A laminin receptor on red blood cells and in various tissues. Connect. Tissue Res. 2005, 46, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.F.; Mallinson, G.; Holmes, C.H.; Houlihan, J.M.; Simpson, K.L.; Mawby, W.J.; Spurr, N.K.; Warne, D.; Barclay, A.N.; Anstee, D.J. The Lutheran blood group glycoprotein, another member of the immunoglobulin superfamily, is widely expressed in human tissues and is developmentally regulated in human liver. Proc. Natl. Acad. Sci. USA 1995, 92, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Rahuel, C.; Le Van Kim, C.; Mattei, M.G.; Cartron, J.P.; Colin, Y. A unique gene encodes spliceoforms of the B-cell adhesion molecule cell surface glycoprotein of epithelial cancer and of the Lutheran blood group glycoprotein. Blood 1996, 88, 1865–1872. [Google Scholar] [PubMed]
- El Nemer, W.; Gane, P.; Colin, Y.; D’Ambrosio, A.M.; Callebaut, I.; Cartron, J.P.; Van Kim, C.L. Characterization of the laminin binding domains of the Lutheran blood group glycoprotein. J. Biol. Chem. 2001, 276, 23757–23762. [Google Scholar]
- El Nemer, W.; Gane, P.; Colin, Y.; Bony, V.; Rahuel, C.; Galacteros, F.; Cartron, J.P.; Le van, K.C. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 1998, 273, 16686–16693. [Google Scholar]
- Udani, M.; Zen, Q.; Cottman, M.; Leonard, N.; Jefferson, S.; Daymont, C.; Truskey, G.; Telen, M.J. Basal cell adhesion molecule/Lutheran protein. The receptor critical for sickle cell adhesion to laminin. J. Clin. Investig. 1998, 101, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.F.; Lee, G.; Spring, F.A.; Willig, T.N.; Peters, L.L.; Gimm, J.A.; Tanner, M.J.; Mohandas, N.; Anstee, D.J.; Chasis, J.A. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind α5 chain-containing human laminin with high affinity. Blood 2001, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Miwa, T.; Tohara, Y.; Hamakubo, T.; Nomizu, M. An antibody to the Lutheran glycoprotein (Lu) recognizing the LU4 blood type variant inhibits cell adhesion to laminin α5. PLoS ONE 2011, 6, e23329. [Google Scholar] [CrossRef] [PubMed]
- Mankelow, T.J.; Burton, N.; Stefansdottir, F.O.; Spring, F.A.; Parsons, S.F.; Pedersen, J.S.; Oliveira, C.L.; Lammie, D.; Wess, T.; Mohandas, N.; et al. The laminin 511/521-binding site on the Lutheran blood group glycoprotein is located at the flexible junction of ig domains 2 and 3. Blood 2007, 110, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Buetow, L.; Flatau, G.; Chiu, K.; Boquet, P.; Ghosh, P. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat. Struct. Biol. 2001, 8, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Aktories, K.; Schmidt, G. Change in substrate specificity of cytotoxic necrotizing factor (CNF) unmasks proteasome-independent down-regulation of constitutively active RhoA. J. Biol. Chem. 2007, 282, 10826–10832. [Google Scholar] [CrossRef] [PubMed]
- Bacher, G.; Szymanski, W.W.; Kaufman, S.L.; Zollner, P.; Blaas, D.; Allmaier, G. Charge-reduced nano electrospray ionization combined with differential mobility analysis of peptides, proteins, glycoproteins, noncovalent protein complexes and viruses. J. Mass Spectrom. 2001, 36, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, P.; Chaar, V.; Picot, J.; Bachir, D.; Habibi, A.; Fauroux, C.; Galacteros, F.; Colin, Y.; Le van, K.C.; El Nemer, W. Decreased sickle red blood cell adhesion to laminin by hydroxyurea is associated with inhibition of Lu/BCAM protein phosphorylation. Blood 2010, 116, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E.; Rahuel, C.; Wautier, M.P.; El Nemer, W.; Gane, P.; Wautier, J.L.; Cartron, J.P.; Colin, Y.; Le van, K.C. Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin α5. J. Biol. Chem. 2005, 280, 30055–30062. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.; Klein, C.E.; Hogenkamp, V.; Kaufmann, R.; Wienrich, B.G.; Schon, M.P. Basal-cell adhesion molecule (B-CAM) is induced in epithelial skin tumors and inflammatory epidermis, and is expressed at cell-cell and cell-substrate contact sites. J. Investig. Dermatol. 2000, 115, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, A.; Cardaci, S.; Lamba, S.; Oddo, D.; Marchio, C.; Cassoni, P.; Amoreo, C.A.; Corti, G.; Testori, A.; Bussolino, F.; et al. BCAM and LAMA5 mediate the recognition between tumor cells and the endothelium in the metastatic spreading of KRAS-mutant colorectal cancer. Clin. Cancer Res. 2016, 22, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Petoukhov, M.V.; Franke, D.; Shkumatov, A.V.; Tria, G.; Kikhney, A.G.; Gajda, M.; Gorba, C.; Mertens, H.D.; Konarev, P.V.; Svergun, D.I. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 2012, 45, 342–350. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reppin, F.; Cochet, S.; El Nemer, W.; Fritz, G.; Schmidt, G. High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein. Toxins 2018, 10, 3. https://doi.org/10.3390/toxins10010003
Reppin F, Cochet S, El Nemer W, Fritz G, Schmidt G. High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein. Toxins. 2018; 10(1):3. https://doi.org/10.3390/toxins10010003
Chicago/Turabian StyleReppin, Franziska, Sylvie Cochet, Wassim El Nemer, Günter Fritz, and Gudula Schmidt. 2018. "High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein" Toxins 10, no. 1: 3. https://doi.org/10.3390/toxins10010003




