Is Erythrocyte Protoporphyrin a Better Single Screening Test for Iron Deficiency Compared to Hemoglobin or Mean Cell Volume in Children and Women?
Abstract
:1. Introduction
2. Materials and Methods
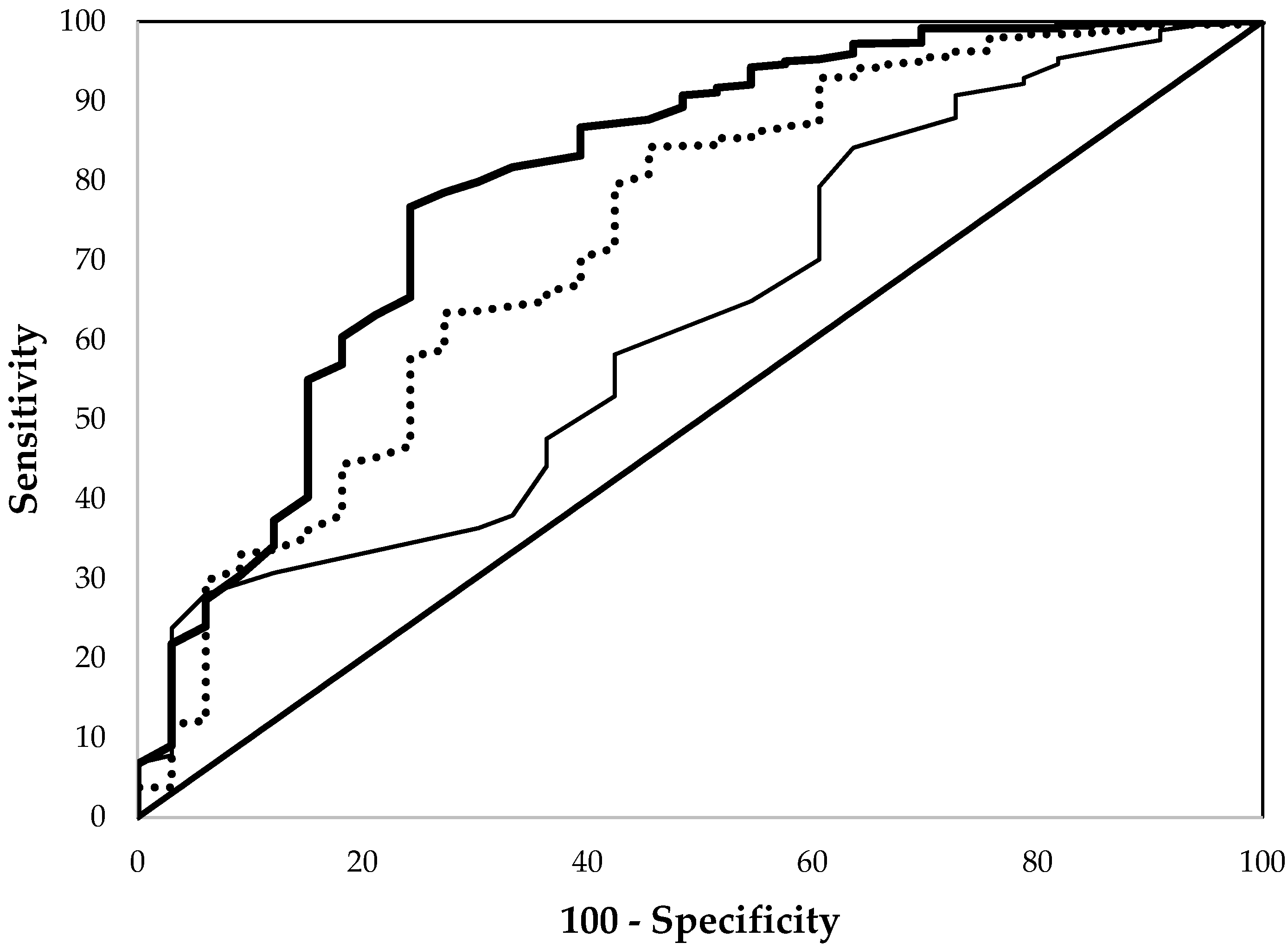
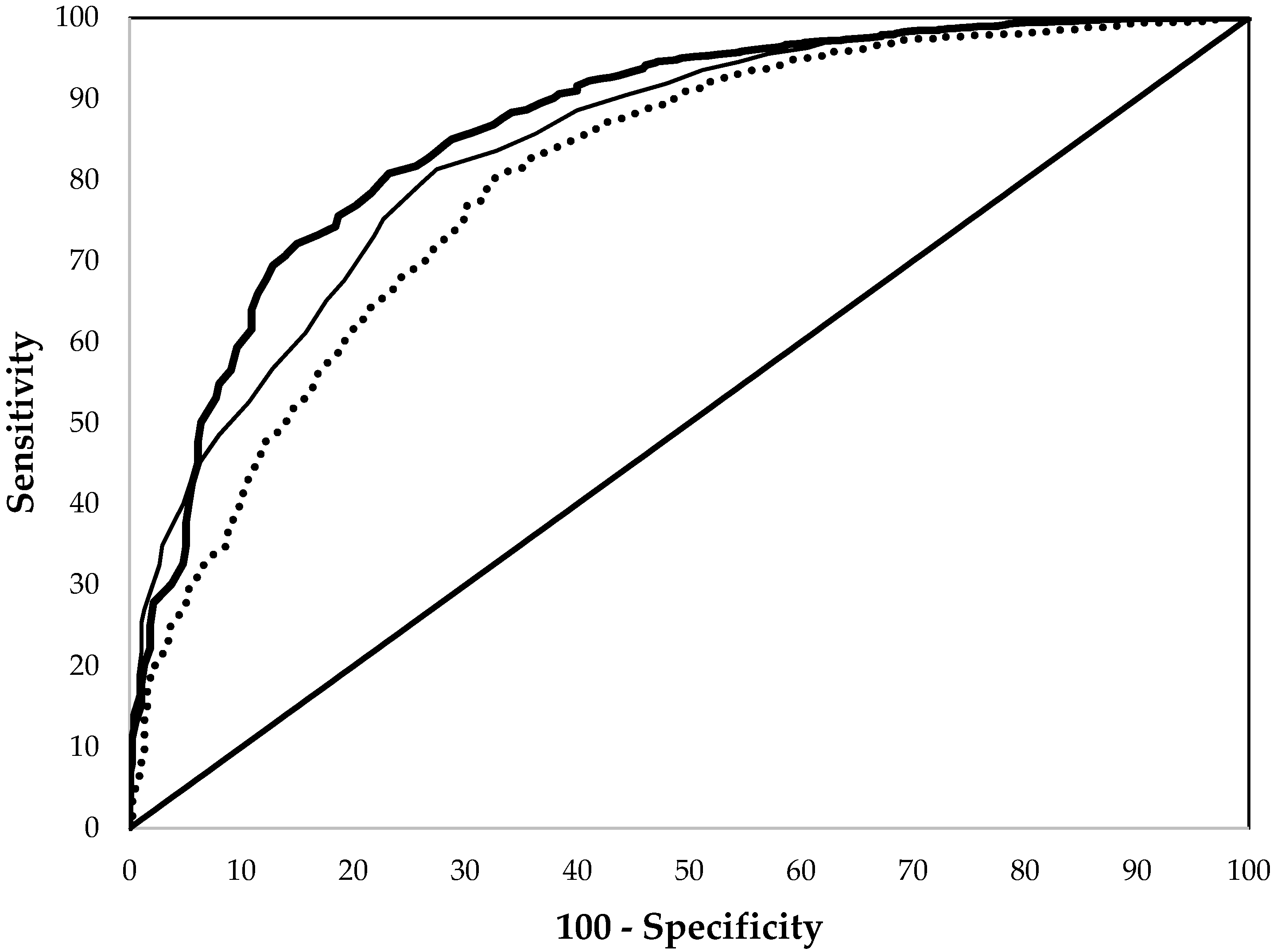
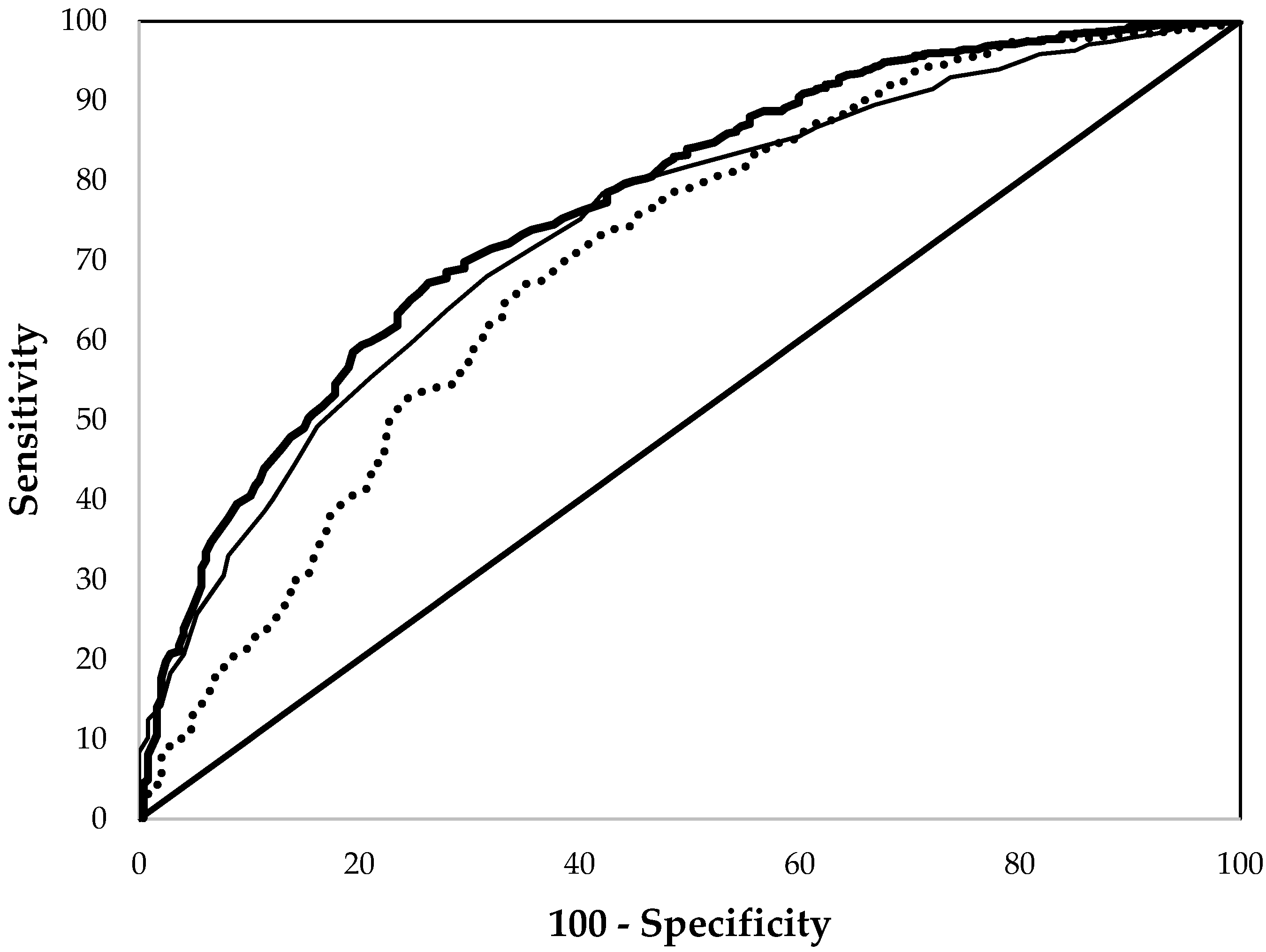
3. Results
4. Discussion and Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ID | iron deficiency |
| Hb | hemoglobin |
| EP | erythrocyte protoporphyrin |
| MCV | mean cell volume |
| TS | transferrin saturation |
| SF | serum ferritin |
| sTfR | serum transferrin receptor |
| ZnPP | zinc protoporphyrin |
| TBI | total body iron |
| CRP | C-reactive protein |
| NHANES | National Health and Nutrition Examination Surveys |
| ROC | receiver operating characteristic |
References
- World Health Organization. Micronutrient Deficiencies: ID Anaemia. Available online: www.who.int/nutrition/topics/ida/en/ (accessed on 19 March 2016).
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Binkin, N.J.; Yip, R. When is anemia screening of value in detecting ID? In Recent Knowledge on Iron and Folate Deficiencies in the World, Proceedings of the Aspects Actuels des Carences en Fer et en Folates Dans le Monde, Paris, France, 11–13 July 1989; Hercberg, S., Galan, P., Dupin, H., Eds.; Institut National de la Santé et la Recherche Médicale: Paris, France, 1990; pp. 137–146. [Google Scholar]
- U.S. Preventive Services Task Forcee. Guide to Clinical Preventive Services, 2nd ed.; Department of Health and Human Services: Washington, DC, USA, 1996.
- Centers for Disease Control and Prevention. Recommendations to prevent and control ID in the United States. MMWR 1998, 47, 1–29. [Google Scholar]
- Blumberg, W.E.; Eisinger, J.; Lamola, A.A.; Zuckerman, D.M. The hematofluorometer. Clin. Chem. 1977, 23, 270–274. [Google Scholar] [PubMed]
- Looker, A.C.; Gunter, E.W.; Johnson, C.L. Methods to assess iron status in various NHANES surveys. Nutr. Rev. 1995, 53, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Pilch, S.M.; Senti, F.R. Assessment of the Iron Nutritional Status of the U.S. Population Based on Data Collected in the Second National Health and Nutrition Examination Survey, 1976–80; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 1984. [Google Scholar]
- Hennig, G.; Homann, C.; Teksan, I.; Hasbargen, U.; Hasmüller, S.; Holdt, L.M.; Khaled, N.; Sroka, R.; Stauch, T.; Stepp, H.; et al. Non-invasive detection of ID by fluorescence measurement of erythrocyte zinc protoporphyrin in the lip. Nat. Commun. 2016, 7, 10776. [Google Scholar] [CrossRef] [PubMed]
- Expert Scientific Working Group. Summary of a report on assessment of the iron nutritional status of the United States. Am. J. Clin. Nutr. 1985, 42, 1318–1330. [Google Scholar]
- Looker, A.C.; Dallman, P.R.; Corroll, M.D.; Gunter, E.W.; Johnson, C.L. Prevalence of ID in the United States. JAMA 1997, 277, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Skikne, B.S.; Flowers, C.H.; Cook, J.D. Serum transferrin receptor: A quantitative measure of tissue ID. Blood 1990, 75, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cook, J.D.; Lacher, D.A.; Beard, J.L.; Lynch, S.R.; Grummer-Strawn, L.M. Assessment of ID in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am. J. Clin. Nutr. 2009, 89, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cusick, S.E.; Lacher, D.A.; Grummer-Strawn, L.M. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey, 1999–2006. Am. J. Clin. Nutr. 2011, 93, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Healthy People 2020; Office of Disease Prevention and Health Promotion: Washington, DC, USA, 2010.
- Mei, Z.; Parvanta, I.; Cogswell, M.E.; Gunter, E.W.; Grummer-Strawn, L.M. Erythrocyte protoporphyrin or hemoglobin: Which is a better screening test for ID in children and women? Am. J. Clin. Nutr. 2003, 77, 1229–1233. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention, National Center for Health Statistics. 1999–2000 National Health and Nutrition Examination Survey (NHANES). Available online: http://wwwn.cdc.gov/nchs/nhanes/nhanes99_00/nhanes99_00.htm (accessed 19 March 2016).
- Centers for Disease Control and Prevention, National Center for Health Statistics. 2001–2002 National Health and Nutrition Examination Survey (NHANES). Available online: http://wwwn.cdc.gov/nchs/nhanes/nhanes01_02/nhanes01_02.htm (accessed 19 March 2016).
- Centers for Disease Control and Prevention, National Center for Health Statistics. 2003–2004 National Health and Nutrition Examination Survey (NHANES). Available online: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/nhanes03_04.htm (accessed 19 March 2016).
- Centers for Disease Control and Prevention, National Center for Health Statistics. 2005–2006 National Health and Nutrition Examination Survey (NHANES). Available online: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm (accessed 19 March 2016).
- Kolbe-Busch, S.; Lotz, J.; Hafner, G.; Blanckaert, N.J.; Claeys, G.; Togni, G.; Carlsen, J.; Röddiger, R.; Thomas, L. Multicenter evaluation of a fully mechanized soluble transferrin assay on the Hitachi and Cobas Integra analyzers. The determination of reference ranges. Clin. Chem. Lab. Med. 2002, 40, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sassa, S.; Granick, J.L.; Granick, S.; Kappas, A.; Levere, R.D. Studies in lead poisoning: I. Microanalysis of erythrocyte protoporphyrin levels by spectrophotometry in the detection of chronic lead intoxication in the subclinical range. Biochem. Med. 1973, 8, 135–148. [Google Scholar] [CrossRef]
- Chisolm, J., Jr.; Brown, D.H. Micro-scale photofluorometric determination of “free erythrocyte pophyrin” (protoporphyrin IX). Clin Chem. 1975, 21, 1669–1682. [Google Scholar] [PubMed]
- NCCLS. Erythrocyte Protoporphyrin Testing; Approved Guideline (National Committee on Clinical Laboratory Standards, 1996). Available online: http://shop.clsi.org/site/Sample_pdf/C42A_sample.pdf (accessed on 19 March 2016).
- Flowers, C.H.; Skikne, B.S.; Covell, A.M.; Cook, J.D. The clinical measurement of serum transferrin receptor. J. Lab. Clin. Med. 1989, 114, 368–3677. [Google Scholar] [PubMed]
- Mirel, L.B.; Curtin, L.R.; Gahche, J.; Burt, V. Characteristics of pregnant women from the 2001–06 National Health and Nutrition Examination Survey. In Proceedings of the Joint Statistical Meeting, Section on Government Statistics, Salt Lake City, UT, USA, 29 July–2 August 2007; pp. 2592–2601. [Google Scholar]
- Metz, C.E. Basic principle of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Yip, R.; Dallman, P.R. The roles of inflammation and ID as causes of anemia. Am. J. Clin. Nutr. 1988, 48, 1295–1300. [Google Scholar] [PubMed]
- Fleming, D.J.; Jacques, P.F.; Dallal, G.E.; Tucker, K.L.; Wilson, P.W.F.; Wood, R.J. Dietary determinants of iron stores in a free-living elderly population: The Framingham Heart Study. Am. J. Clin. Nutr. 1998, 67, 722–733. [Google Scholar] [PubMed]
- Labbe, R.F.; Vreman, H.J.; Stevenson, D.K. Zinc protoporphyrin: A metabolite with a mission. Clin. Chem. 1999, 45, 2060–2072. [Google Scholar] [PubMed]
- Dallman, P.R.; Siimes, M.A. Percentile curves for hemoglobin and red cell volume in infancy and childhood. J. Pediatr. 1979, 94, 26–31. [Google Scholar] [CrossRef]
- Crowell, R.; Ferris, A.M.; Wood, R.J.; Joyce, P.; Slivka, H. Comparative effectiveness of zinc protoporphyrin and hemoglobin concentrations in identifying ID in a group of low-income, preschool-aged children: Practical implications of recent illness. Pediatrics 2006, 118, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Lamola, A.A.; Yamane, T. Zinc protoporphyrin in the erythrocytes of patients with lead intoxication and ID anemia. Science 1974, 186, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Labbe, R.F. History and background of protoporphyrin testing. Clin. Chem. 1977, 23, 256–259. [Google Scholar] [PubMed]
- Dallman, P.R.; Siimes, M.A.; Stekel, A. ID in infant and childhood. Am. J. Clin. Nutr. 1980, 33, 86–118. [Google Scholar] [PubMed]
- Yip, R. The changing characteristics of childhood iron nutritional status in the United States. In Dietary Iron: Birth to Two Years; Filer, L.J., Jr., Ed.; Raven Press: New York, NY, USA, 1989; pp. 37–61. [Google Scholar]
- Martin, C.J.; Werntz, C.L.; Ducatman, A.M. The interpretation of zinc protoporphyrin changes in lead intoxication: A case report and review of the literature. Occup. Med. 2004, 54, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Perseu, L.; Moi, P.; Asunis, I.; Cabriolu, A.; Maccioni, L.; Demartis, F.R.; Manunza, L.; Cao, A.; Galanello, R. Compound heterozygosity for KLF1 mutations associated with remarkable increase of fetal hemoglobin and red cell protoporphyrin. Haematologica 2011, 96, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; De Falco, L.; Beaumont, C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica 2009, 94, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Hastka, J.; Lasserre, J.J.; Schwarzbeck, A.; Strauch, M.; Hehlmann, R. Zinc protoporphyrin: A screening parameter of iron metabolism. Onkologie 1991, 14, 62–63. [Google Scholar]
- Besur, S.; Hou, W.; Schmeltzer, P.; Bonkovsky, H.L. Clinically important features of porphyrin and heme metabolism and the porphyrias. Metabolites 2014, 4, 977–1006. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E.; Pulakhandam, U.R.; Billett, H.H.; Nagel, R.L. Blood zinc protoporphyrin is elevated only in sickle cell patients with low fetal hemoglobin. Am. J. Hematol. 1991, 36, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.M.; Lightsey, A.L.; Schanberger, J.E. The micromeasurement of free erythrocyte protoporphyrin as a means of differentiating alpha thalassemia trait from ID anemia. J. Pediatr. 1975, 86, 539–541. [Google Scholar] [CrossRef]
- Stockman, J.A.; Weiner, L.S.; Simon, G.E.; Stuart, M.J.; Oski, F.A. The measurement of free erythrocyte porphyrin (FEP) as a simple means of distinguishing ID from beta-thalassemia trait in subjects with microcytosis. J. Lab. Clin. Med. 1975, 85, 113–119. [Google Scholar] [PubMed]
- Mwangi, M.N.; Maskey, S.; Andang, P.E.; Shinali, N.K.; Roth, J.M.; Trijsburg, L.; Mwangi, A.M.; Zuilhof, H.; van Lagen, B.; Savelkoul, H.F.; et al. Diagnostic utility of zinc protoporphyrin to detect ID in Kenyan pregnant women. BMC Med. 2014, 12, 229. [Google Scholar] [CrossRef]
- Tillyer, M.L.; Tillyer, C.R. Zinc protoporphyrin assays in patients with alpha and beta thalassaemia trait. J. Clin. Pathol. 1994, 47, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.F.; Lilleyman, J.S.; Steel, G.J.; Bellamy, G.J. Usefulness of red cell zinc protoporphyrin concentration in the investigation of microcytosis in children. Pediatr. Hematol. Oncol. 1995, 12, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.A.; Felgenhauer, J.; Detter, J.C.; Labbe, R.F. Elevated zinc protoporphyrin associated with thalassemia trait and hemoglobin E. J. Pediatr. 1996, 129, 105–110. [Google Scholar] [CrossRef]
- Hinchliffe, R.F.; Vora, A.J.; Lennard, L. An assessment of methods used in the investigation of iron status: Findings in a population of young British South Asian children. J. Clin. Pathol. 2016, 69, 345–351. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Centers of Disease Control and Prevention. Assessing the iron status of populations: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
| Children (n = 853) | Non-Pregnant Women (n = 3104) | Pregnant Women (n = 1141) | |
|---|---|---|---|
| % of female | 48.8 | 100 | 100 |
| Age (years) 2 | 4.0 ± 0.8 | 28.4 ± 11.1 | 26.3 ± 5.7 |
| % of Non-Hispanic white | 27.6 | 39.0 | 44.0 |
| % of Non-Hispanic black | 31.0 | 28.3 | 15.6 |
| % of Mexican-American | 29.1 | 24.4 | 29.7 |
| Others | 12.3 | 8.3 | 10.7 |
| EP (μmol/L) 2 | 0.91 ± 0.36 | 1.13 ± 0.72 | 1.26 ± 0.62 |
| Hb (g/dL) 2 | 12.77 ± 0.80 | 13.40 ± 1.17 | 12.36 ± 1.04 |
| MCV (fL) 2 | 82.05 ± 4.34 | 88.09 ± 5.98 | 89.40 ± 5.42 |
| SF (μg/L) 2 | 29.70 ± 1.72 | 34.26 ± 2.51 | 19.58 ± 2.54 |
| sTfR (mg/L) 2 | 4.21 ± 1.24 | 3.62 ± 1.41 | 3.35 ± 1.42 |
| TBI (mg/kg) 2 | 3.91 ± 2.22 | 4.92 ± 4.04 | 3.17 ± 4.10 |
| Lead (μg/dL) | 2.01 ± 1.41 | 1.10 ± 0.86 | 0.94 ± 0.75 |
| CRP (mg/L) 2 | 0.04 ± 4.06 | 0.15 ± 4.42 | 0.48 ± 2.74 |
| % anemia 3 | 1.4 | 9.1 | 5.6 |
| % TBI < 0 mg/kg | 3.9 | 12.1 | 21.6 |
| Children (n = 853) 2 | Non-Pregnant Women (n = 3104) 2 | Pregnant Women (n = 1141) 2 | |
|---|---|---|---|
| Total sample | |||
| EP | 0.799 ± 0.046 a | 0.864 ± 0.010 a | 0.766 ± 0.016 a |
| Hb | 0.624 ± 0.050 b | 0.843 ± 0.011 a | 0.740 ± 0.017 a |
| MCV | 0.728 ± 0.050 a | 0.802 ± 0.013 b | 0.700 ± 0.020 b |
| Male | |||
| EP | 0.787 ± 0.066 c | - | - |
| Hb | 0.635 ± 0.067 d | - | - |
| MCV | 0.768 ± 0.067 c | - | - |
| Female | |||
| EP | 0.825 ± 0.054 a | 0.864 ± 0.010 a | 0.766 ± 0.016 a |
| Hb | 0.607 ± 0.076 b | 0.843 ± 0.011 a | 0.740 ± 0.017 a |
| MCV | 0.669 ± 0.067 b | 0.802 ± 0.013 b | 0.700 ± 0.020 b |
| Non-Hispanic white | |||
| EP | 0.806 ± 0.069 c | 0.878 ± 0.019 a | 0.778 ± 0.028 a |
| Hb | 0.599 ± 0.115 d | 0.878 ± 0.018 a | 0.783 ± 0.027 a |
| MCV | 0.730 ± 0.090 c | 0.836 ± 0.022 a | 0.726 ± 0.033 a |
| Non-Hispanic black | |||
| EP | 0.665 ± 0.127 a | 0.882 ± 0.018 a | 0.828 ± 0.034 a |
| Hb | 0.495 ± 0.106 a | 0.843 ± 0.019 a | 0.667 ± 0.042 b |
| MCV | 0.575 ± 0.117 a | 0.761 ± 0.025 b | 0.653 ± 0.049 b |
| Mexican American | |||
| EP | 0.821 ± 0.066 c | 0.829 ± 0.020 a | 0.744 ± 0.030 a |
| Hb | 0.677 ± 0.071 d | 0.830 ± 0.021 a | 0.753 ± 0.029 a |
| MCV | 0.781 ± 0.082 c | 0.815 ± 0.022 a | 0.688 ± 0.034 a |
| 1st trimester | |||
| EP | - | - | 0.873 ± 0.053 c |
| Hb | - | - | 0.764 ± 0.066 c |
| MCV | - | - | 0.730 ± 0.080 d |
| 2nd trimester | |||
| EP | - | - | 0.744 ± 0.032 a |
| Hb | - | - | 0.746 ± 0.031 a |
| MCV | - | - | 0.720 ± 0.036 a |
| 3rd trimester | |||
| EP | - | - | 0.715 ± 0.028 a |
| Hb | - | - | 0.722 ± 0.027 a |
| MCV | - | - | 0.742 ± 0.027 a |
| Children (n = 762) 2 | Non-Pregnant Women (n = 2746) 2 | Pregnant Women (n = 684) 2 | |
|---|---|---|---|
| EP | 0.785 ± 0.049 a | 0.864 ± 0.011 a | 0.771 ± 0.022 a |
| Hb | 0.596 ± 0.054 b | 0.841 ± 0.011 a | 0.743 ± 0.022 a |
| MCV | 0.703 ± 0.052 a | 0.802 ± 0.014 b | 0.698 ± 0.026 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Z.; Flores-Ayala, R.C.; Grummer-Strawn, L.M.; Brittenham, G.M. Is Erythrocyte Protoporphyrin a Better Single Screening Test for Iron Deficiency Compared to Hemoglobin or Mean Cell Volume in Children and Women? Nutrients 2017, 9, 557. https://doi.org/10.3390/nu9060557
Mei Z, Flores-Ayala RC, Grummer-Strawn LM, Brittenham GM. Is Erythrocyte Protoporphyrin a Better Single Screening Test for Iron Deficiency Compared to Hemoglobin or Mean Cell Volume in Children and Women? Nutrients. 2017; 9(6):557. https://doi.org/10.3390/nu9060557
Chicago/Turabian StyleMei, Zuguo, Rafael C. Flores-Ayala, Laurence M. Grummer-Strawn, and Gary M. Brittenham. 2017. "Is Erythrocyte Protoporphyrin a Better Single Screening Test for Iron Deficiency Compared to Hemoglobin or Mean Cell Volume in Children and Women?" Nutrients 9, no. 6: 557. https://doi.org/10.3390/nu9060557




