Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Cell Culture
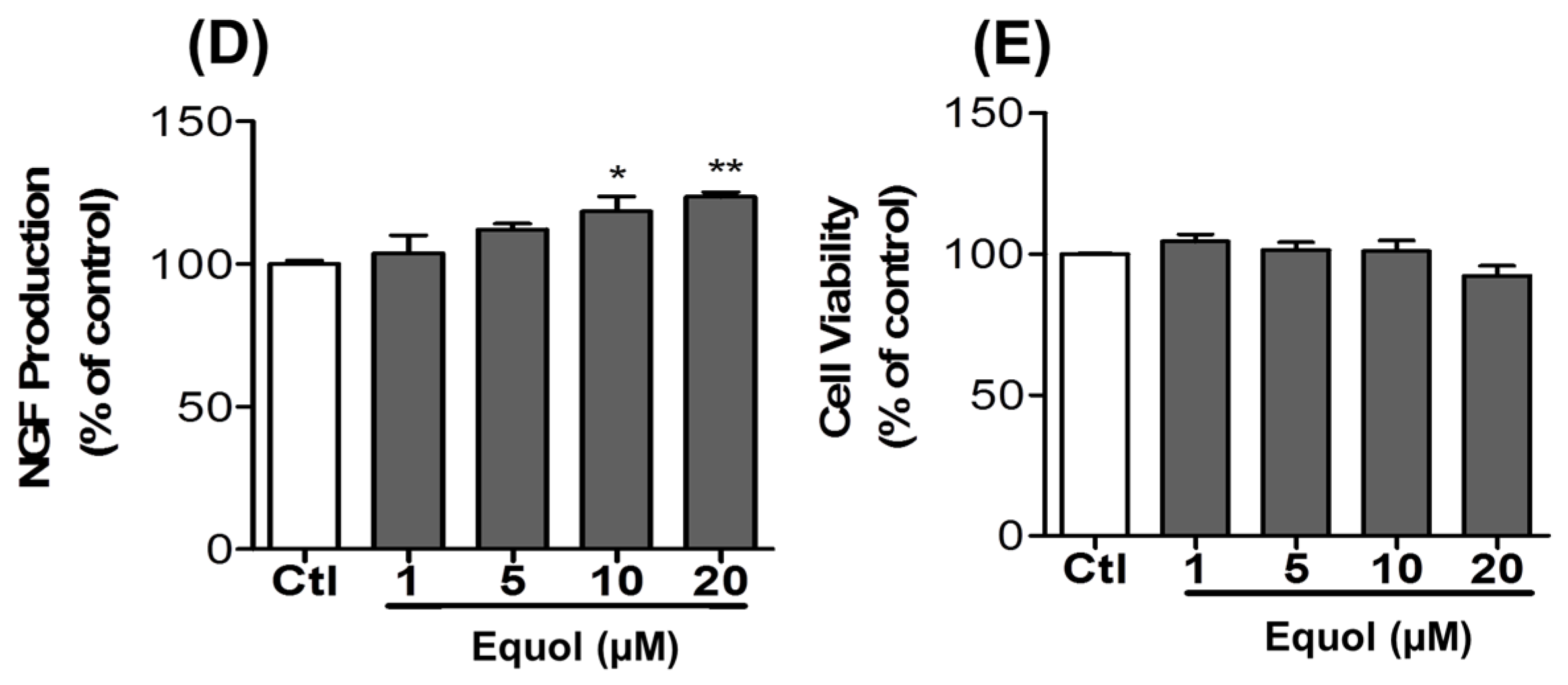
2.3. Cell Viability Assay
2.4. Nitric Oxide (NO) and Proinflammatory Cytokine Measurement
2.5. Measurement of PGE-2, TNF-α, and IL-6 Production
2.6. NF-κB Assay
2.7. Western Blot Analysis
2.8. Neurite Outgrowth Assay
2.9. NGF Assay
2.10. Statistical Analysis
3. Results
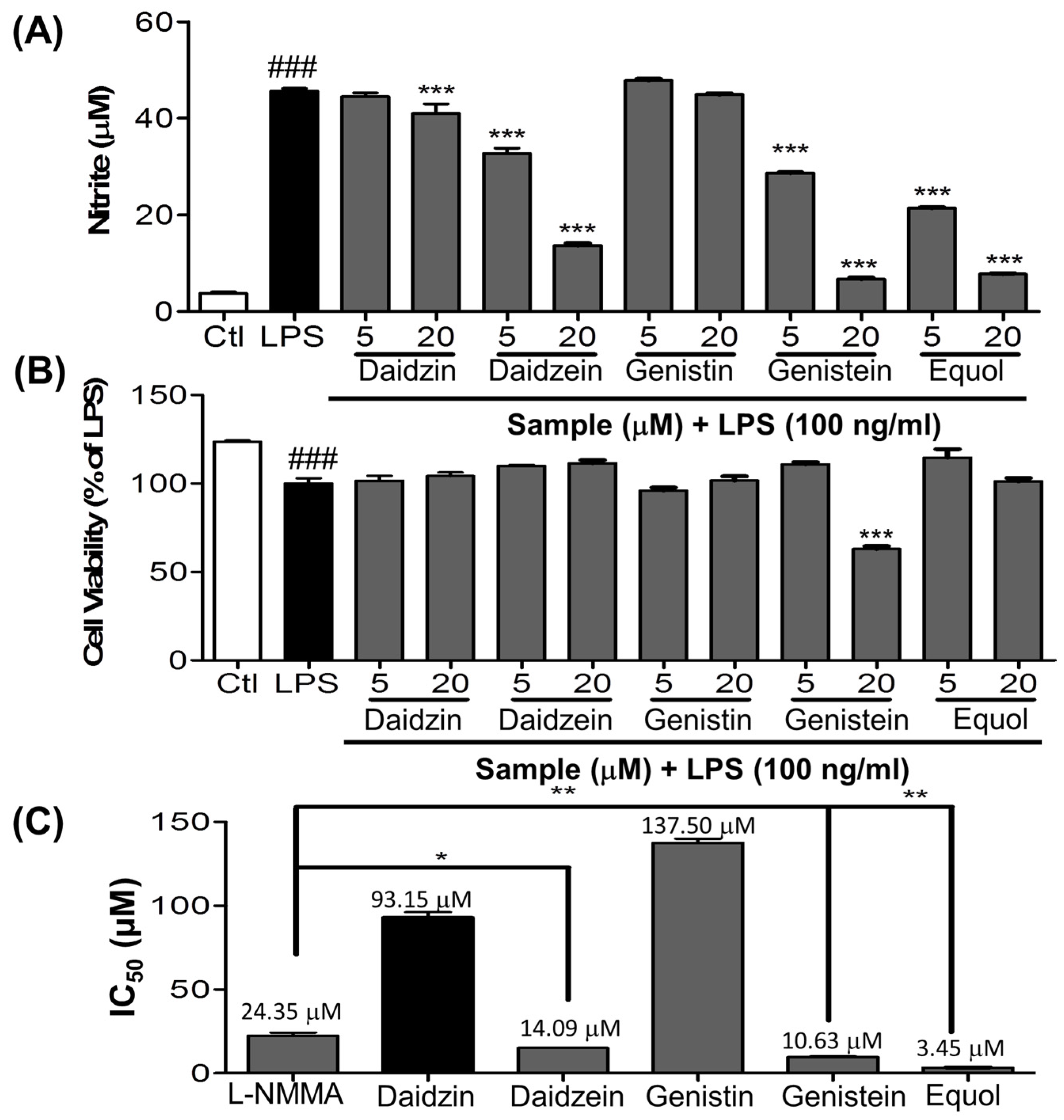
3.1. Effect of Daidzein and Its Derivatives on NO Production in LPS-Stimulated BV-2 Cells
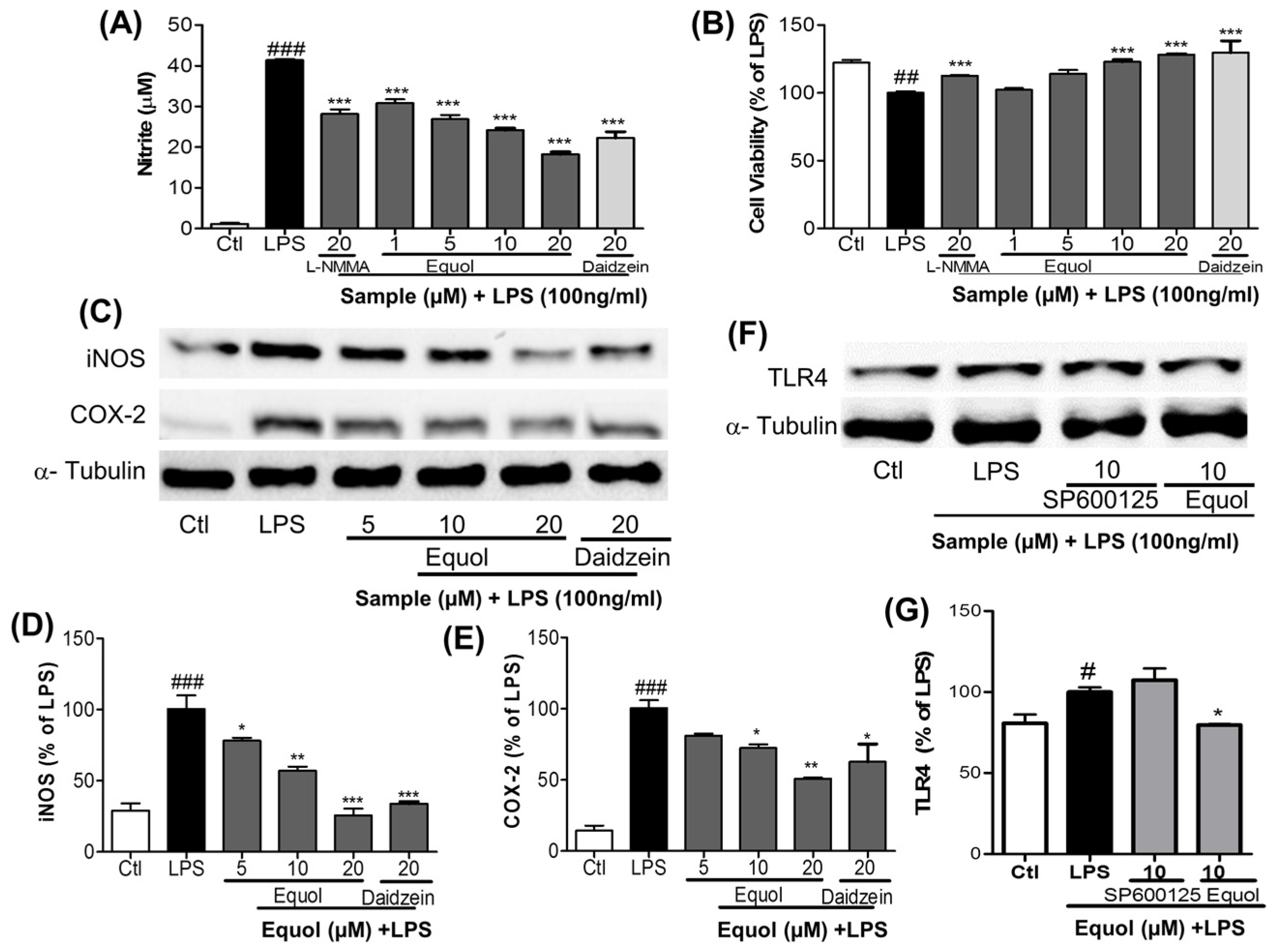
3.2. Dose Response Effect of Equol on NO Production, iNOS and COX-2 Expression and TLR4 Inactivation in LPS-Stimulated BV-2 Cells
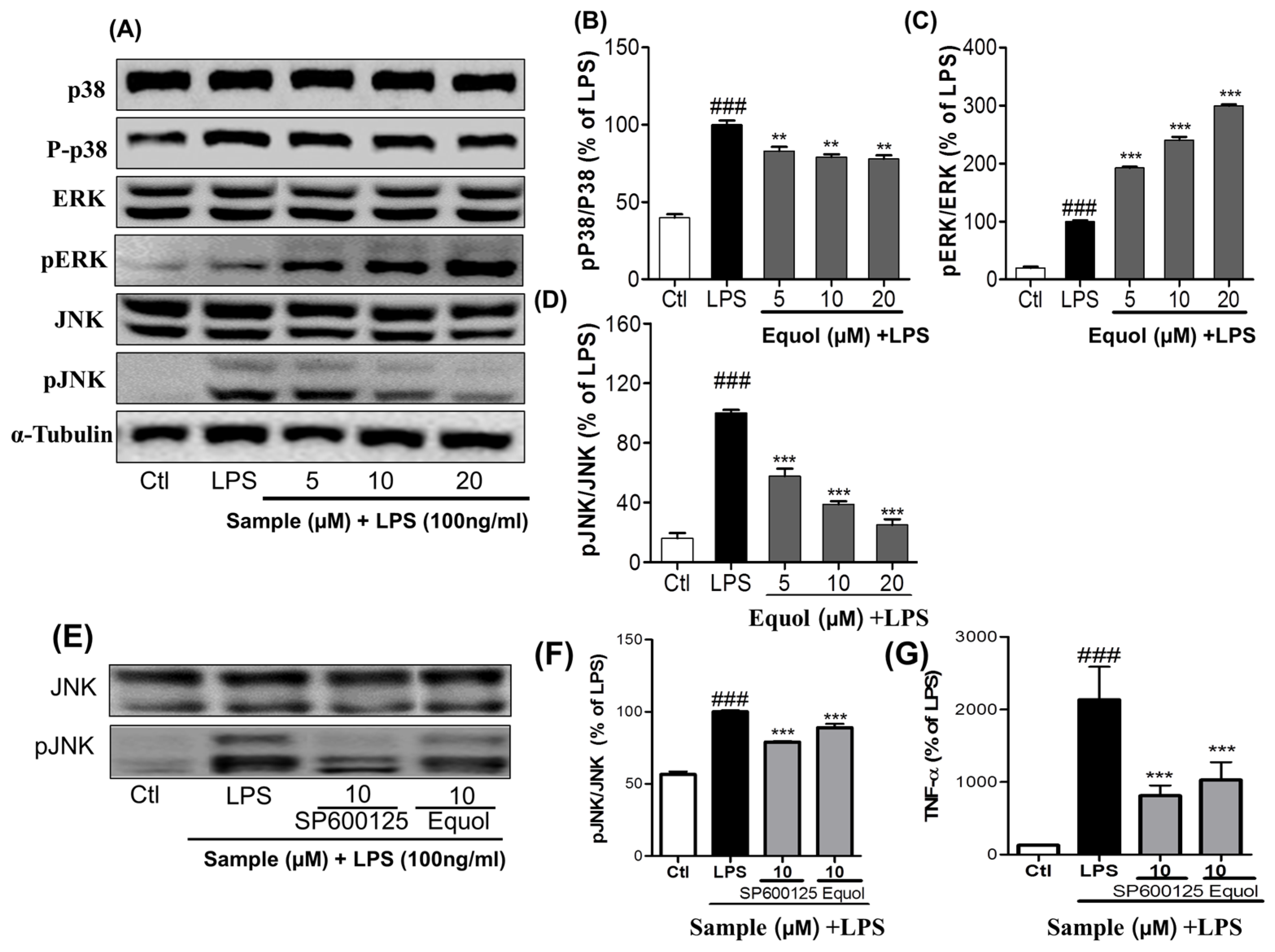
3.3. Effect of Equol on LPS-Induced MAPK Signaling in LPS-Stimulated BV-2 Cells
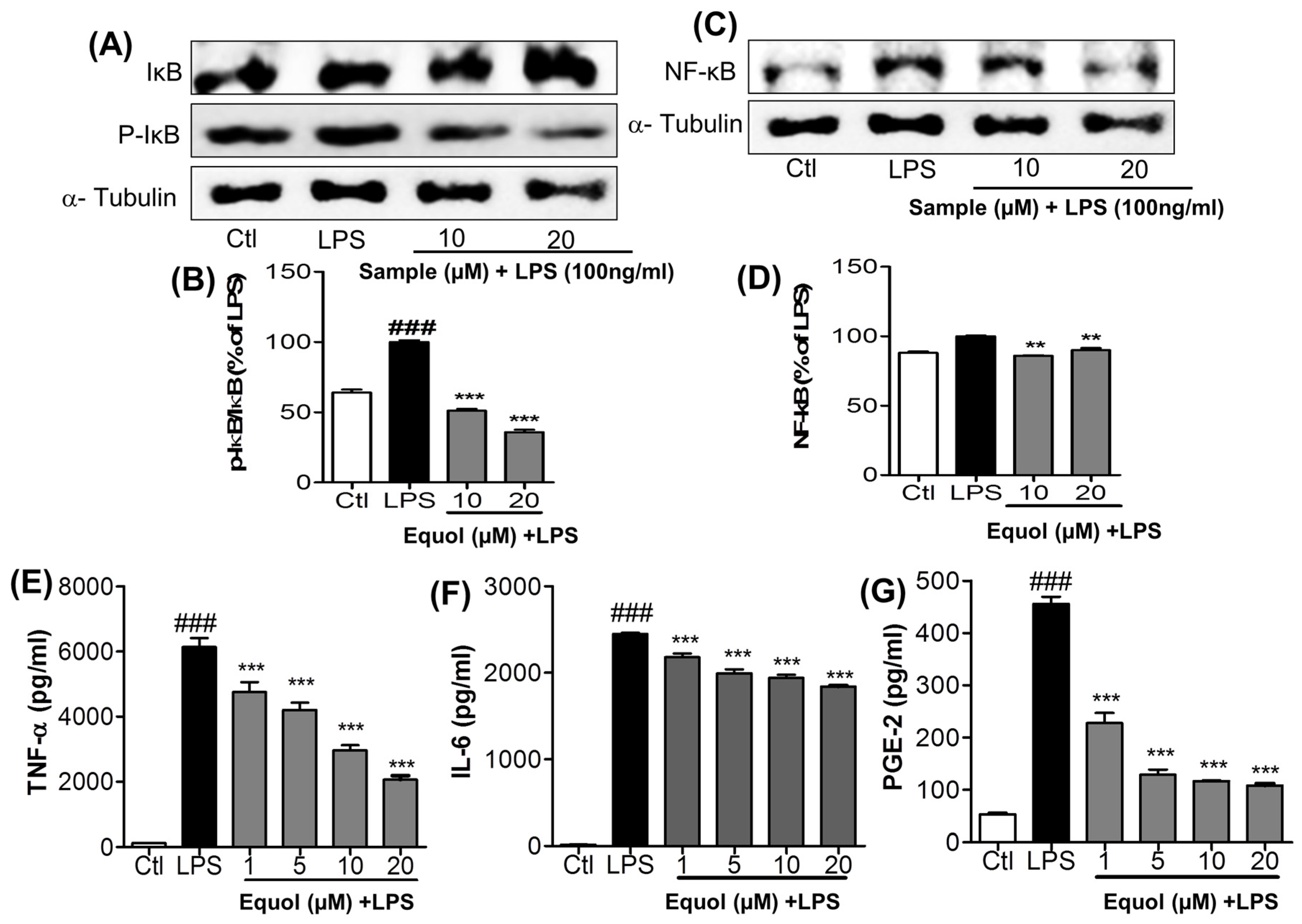
3.4. Effect of Equol on LPS-Induced NF-κB Activation in Murine Microglia Cells
3.5. Effect of Equol on TNF-α, IL-6, andPGE2 Production in LPS-Stimulated BV-2 Cells
3.6. Effect of Equol on Activated Microglia-Induced Neurotoxicity in N2a Cells
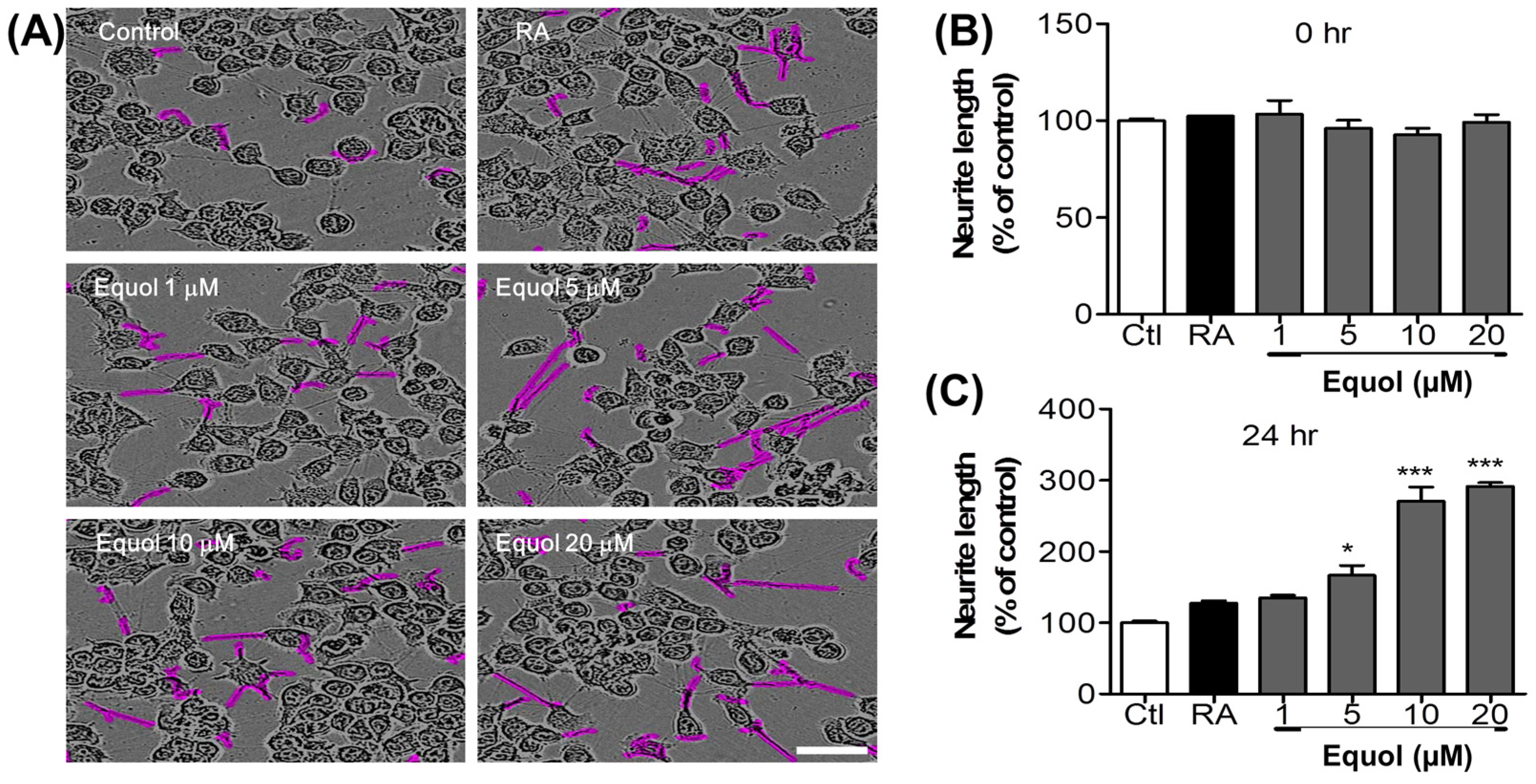
3.7. Effect of Equol on Neurite Outgrowth in N2a Cells
3.8. Effect of Equol on NGF Production in C6 Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ullah, M.F.; Bhat, S.H.; Husain, E.; Abu-Duhier, F.; Hadi, S.M.; Sarkar, F.H.; Ahmad, A. Pharmacological Intervention through Dietary Nutraceuticals in Gastrointestinal Neoplasia. Crit. Rev. Food Sci. Nutr. 2016, 56, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Fura, A. Role of pharmacologically active metabolites in drug discovery and development. Drug Discov. Today 2006, 11, 133–142. [Google Scholar] [CrossRef]
- Howes, M.J.; Perry, E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging 2011, 28, 439–468. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhou, X.; Zuo, L.; Wang, H.; Yu, D. Identification and functional characterization of the sulfate transporter gene GmSULTR1;2b in soybean. BMC Genom. 2016, 17, 373. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Sunita, P.; Pattanayak, S.P. Phytoestrogens in postmenopausal indications: A theoretical perspective. Pharmacogn. Rev. 2011, 5, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bacciottini, L.; Falchetti, A.; Pampaloni, B.; Bartolini, E.; Carossino, A.M.; Brandi, M.L. Phytoestrogens: Food or drug? Clin. Cases Miner. Bone Metab. 2007, 4, 123–130. [Google Scholar] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, C.Q.; Zhang, Q.; Lou, Y.; Wu, H.J.; Deng, J.C.; Yang, F.; Yang, W.Y. Partial improvements in the flavor quality of soybean seeds using intercropping systems with appropriate shading. Food Chem. 2016, 207, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Ali, S.; Philip, P.A.; Wozniak, A.; Sarkar, F.H. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer 2009, 115, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A.; Redmond, L. Soy phytoestrogens are neuroprotective against stroke-like injury in vitro. Neuroscience 2009, 158, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ding, B.; Yu, H.; Yuan, L.; Xi, Y.; Xiao, R. Genistein alleviates beta-amyloid-induced inflammatory damage through regulating Toll-like receptor 4/nuclear factor kappaB. J. Med. Food 2015, 18, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Zhao, X.; Lindley, S.L.; Heubi, J.E.; King, E.C.; Messina, M.J. Soy isoflavone phase II metabolism differs between rodents and humans: Implications for the effect on breast cancer risk. Am. J. Clin. Nutr. 2011, 94, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Rozman, K.K.; Bhatia, J.; Calafat, A.M.; Chambers, C.; Culty, M.; Etzel, R.A.; Flaws, J.A.; Hansen, D.K.; Hoyer, P.B.; Jeffery, E.H.; et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of soy formula. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2006, 77, 280–397. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Sharma, N.; Blackburn, M.; Vantrease, J.; Gomez-Acevedo, H.; Hennings, L.; Shankar, K.; Cleves, M.A.; Badger, T.M.; Ronis, M.J. Feeding soy protein isolate and treatment with estradiol have different effects on mammary gland morphology and gene expression in weanling male and female rats. Physiol. Genom. 2013, 45, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [PubMed]
- Tousen, Y.; Uehara, M.; Abe, F.; Kimira, Y.; Ishimi, Y. Effects of short-term fructooligosaccharide intake on equol production in Japanese postmenopausal women consuming soy isoflavone supplements: A pilot study. Nutr. J. 2013, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Matthies, A.; Loh, G.; Blaut, M.; Braune, A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J. Nutr. 2012, 142, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Atkinson, C.; Wahala, K.; Lampe, J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S.; Bae, S.M. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 2009, 177, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; de Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Cegiela, U.; Folwarczna, J.; Pytlik, M.; Zgorka, G. Effects of extracts from trifolium medium L. and Trifolium pratense L. on development of estrogen deficiency-induced osteoporosis in rats. Evid. Based Complement. Altern. Med. eCAM 2012, 2012, 921684. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Zhao, X.; Jha, P.; Heubi, J.E.; Brown, N.M. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am. J. Clin. Nutr. 2009, 90, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sullivan, J.C.; Schreihofer, D.A. Dietary genistein and equol (4′, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R871–R877. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M. Isoflavone metabolism and bone-sparing effects of daidzein-metabolites. J. Clin. Biochem. Nutr. 2013, 52, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.; Przedborski, S. Oxidative stress in Parkinson’s disease: A mechanism of pathogenic and therapeutic significance. Ann. N. Y. Acad. Sci. 2008, 1147, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Morale, M.C.; Serra, P.A.; L’Episcopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Gennuso, F.; Giaquinta, G.; Rocchitta, G.; Desole, M.S.; et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: Glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 2006, 138, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.C.; Peterlin, B.L.; Calhoun, A.H. Migraine and estrogen. Curr. Opin. Neurol. 2014, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ravn, S.H.; Rosenberg, J.; Bostofte, E. Postmenopausal hormone replacement therapy—Clinical implications. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 53, 81–93. [Google Scholar] [CrossRef]
- Perez-Cano, F.J.; Castell, M. Flavonoids, Inflammation and Immune System. Nutrients 2016, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Mueller, M.; Jungbauer, A. Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients 2010, 2, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Catley, M.C.; Birrell, M.A.; Hardaker, E.L.; de Alba, J.; Farrow, S.; Haj-Yahia, S.; Belvisi, M.G. Estrogen receptor beta: Expression profile and possible anti-inflammatory role in disease. J. Pharmacol. Exp. Ther. 2008, 326, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-inducedmurine neuroinflammation model: Main features and suitability for pre-clinical assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.W.; McCreedy, S.A. N-nitro-l-arginine and N-monomethyl-l-arginine exhibit a different pattern of inactivation toward the three nitric oxide synthases. Arch. Biochem. Biophys. 1995, 320, 170–176. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.N.; Chi, C.W.; Lin, Y.L.; Chen, C.F.; Shiao, Y.J. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J. Biol. Chem. 2001, 276, 5287–5295. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, Y.; Zhou, D.X.; Zhao, L.M.; Li, G.R.; Deng, X.L. Equol is neuroprotective during focal cerebral ischemia and reperfusion that involves p-Src and gp91(phox). Curr. Neurovasc. Res. 2014, 11, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Nair, B.C.; Gonugunta, V.K.; Zhang, Q.G.; Brenner, A.; Brann, D.W.; Tekmal, R.R.; Vadlamudi, R.K. Therapeutic significance of estrogen receptor beta agonists in gliomas. Mol. Cancer Ther. 2012, 11, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Shin, J.A.; Jung, J.S.; Hyun, J.W.; Van Le, T.K.; Kim, D.H.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Tohidast-Akrad, M.; Smolen, J.S.; Schmid, B.J.; Steiner, C.W.; Bitzan, P.; Zenz, P.; Redlich, K.; Xu, Q.; Steiner, G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 2501–2512. [Google Scholar] [CrossRef]
- Koide, Y.; Ito, A.; Edo, K.; Ishida, N. The biologically active site of neocarzinostatin-chromophore. Chem. Pharm. Bull. 1986, 34, 4425–4428. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.P.; Cheeran, M.C.; Palmquist, J.M.; Hu, S.; Lokensgard, J.R. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J. Neurovirol. 2008, 14, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Schurer, L.; Grogaard, B.; Gerdin, B.; Arfors, K.E. Effects of neutrophil depletion and superoxide dismutase on postischemic hypoperfusion of rat brain. Adv. Neurol. 1990, 52, 57–62. [Google Scholar] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jalabi, W.; Shpargel, K.B.; Farabaugh, K.T.; Dutta, R.; Yin, X.; Kidd, G.J.; Bergmann, C.C.; Stohlman, S.A.; Trapp, B.D. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J. Neurosci. 2012, 32, 11706–11715. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Guo, W.; Xu, J.; Zhang, G.; Wang, W.; Huang, W. Ginsenoside Rb1 attenuates activated microglia-induced neuronal damage. Neural Regen. Res. 2014, 9, 252–259. [Google Scholar] [PubMed]
- Guadagno, J.; Xu, X.; Karajgikar, M.; Brown, A.; Cregan, S.P. Microglia-derived TNFalpha induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. 2013, 4, e538. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Scherer, S.S. Neuronal cadherin (NCAD) increases sensory neurite formation and outgrowth on astrocytes. Neurosci. Lett. 2012, 522, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Lent-Schochet, D.; Pike, C.J. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J. Neuroinflamm. 2014, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Koppula, S.; Kim, I.S.; Kumar, H.; Kim, B.W.; Choi, D.K. The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 2012, 17, 6728–6753. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.S.; Killebrew, D.A.; Clary, G.P.; Meeker, R.B. Opposing Effects of NGF and proNGF on HIV Induced Macrophage Activation. J. Neuroimmune Pharmacol. 2016, 11, 98–120. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. https://doi.org/10.3390/nu9030207
Subedi L, Ji E, Shin D, Jin J, Yeo JH, Kim SY. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients. 2017; 9(3):207. https://doi.org/10.3390/nu9030207
Chicago/Turabian StyleSubedi, Lalita, Eunhee Ji, Dongyun Shin, Jongsik Jin, Joo Hong Yeo, and Sun Yeou Kim. 2017. "Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro" Nutrients 9, no. 3: 207. https://doi.org/10.3390/nu9030207





