A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes?
Abstract
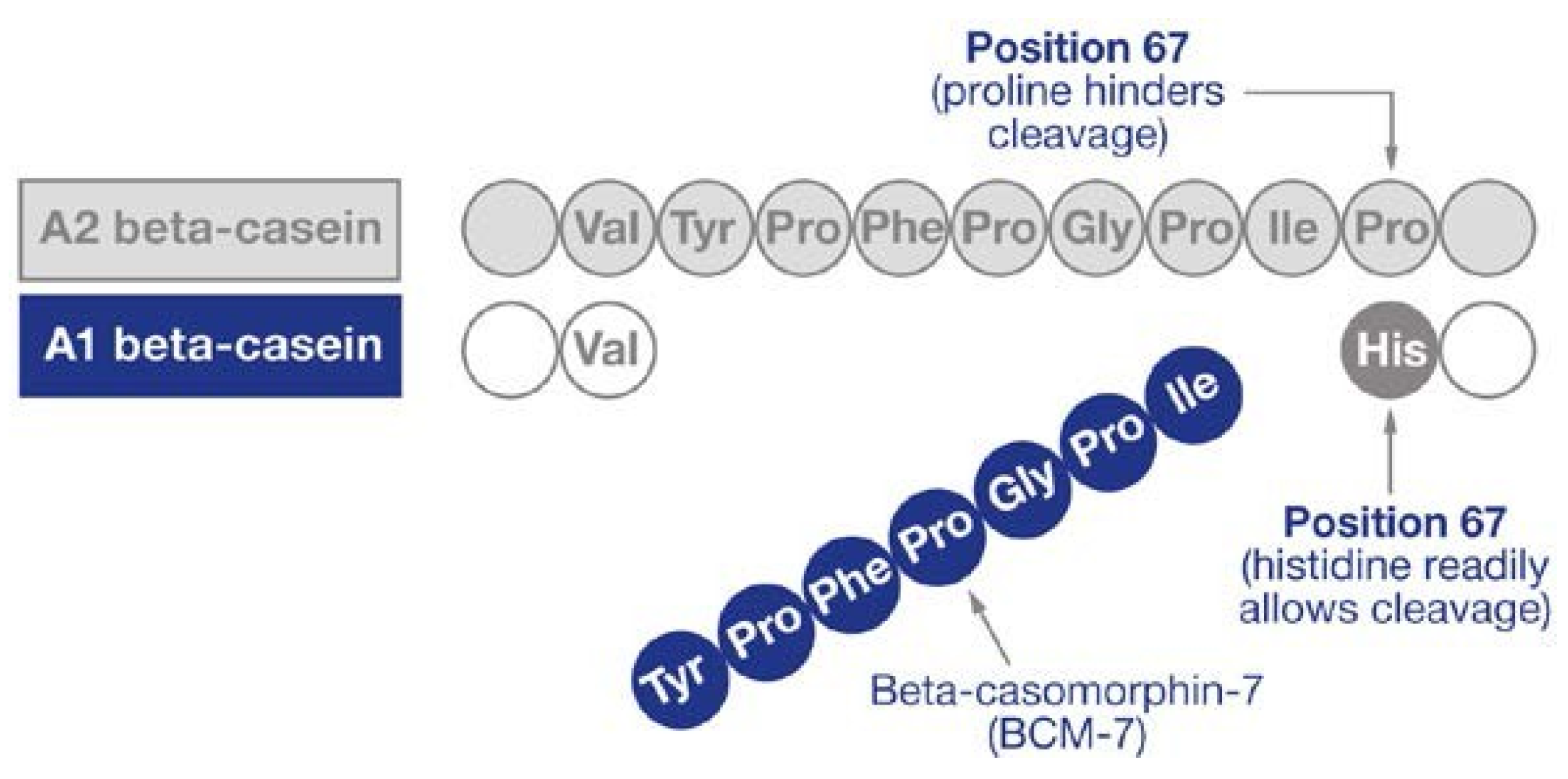
:1. Introduction
2. Materials and Methods
2.1. Participants
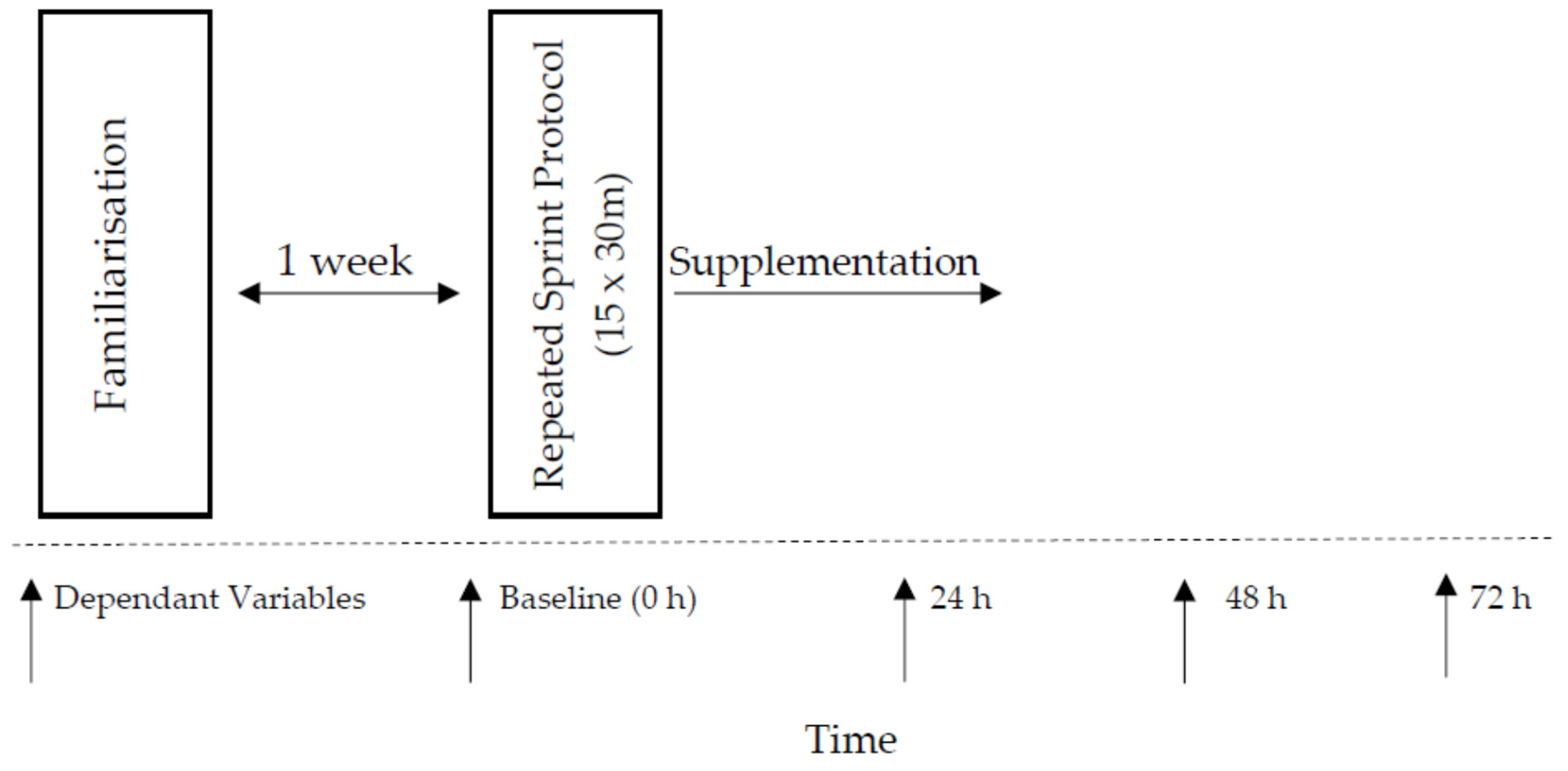
2.2. Study Design
2.3. Nutritional Intervention and Dietary Control
2.4. Baseline Performance Indices
2.4.1. Visual Analogue Scale (Muscle Soreness)
2.4.2. Countermovement Jump (CMJ)
2.4.3. Maximal Voluntary Isometric Contraction (MVIC)
2.4.4. 20 m Sprint Test
2.4.5. Repeated Sprint Protocol
2.5. Statistical Analysis
3. Results
3.1. Group Allocation
3.2. Evidence of Muscle Damage
3.3. Effects of Nutritional Supplement
3.3.1. The 20-m Sprint
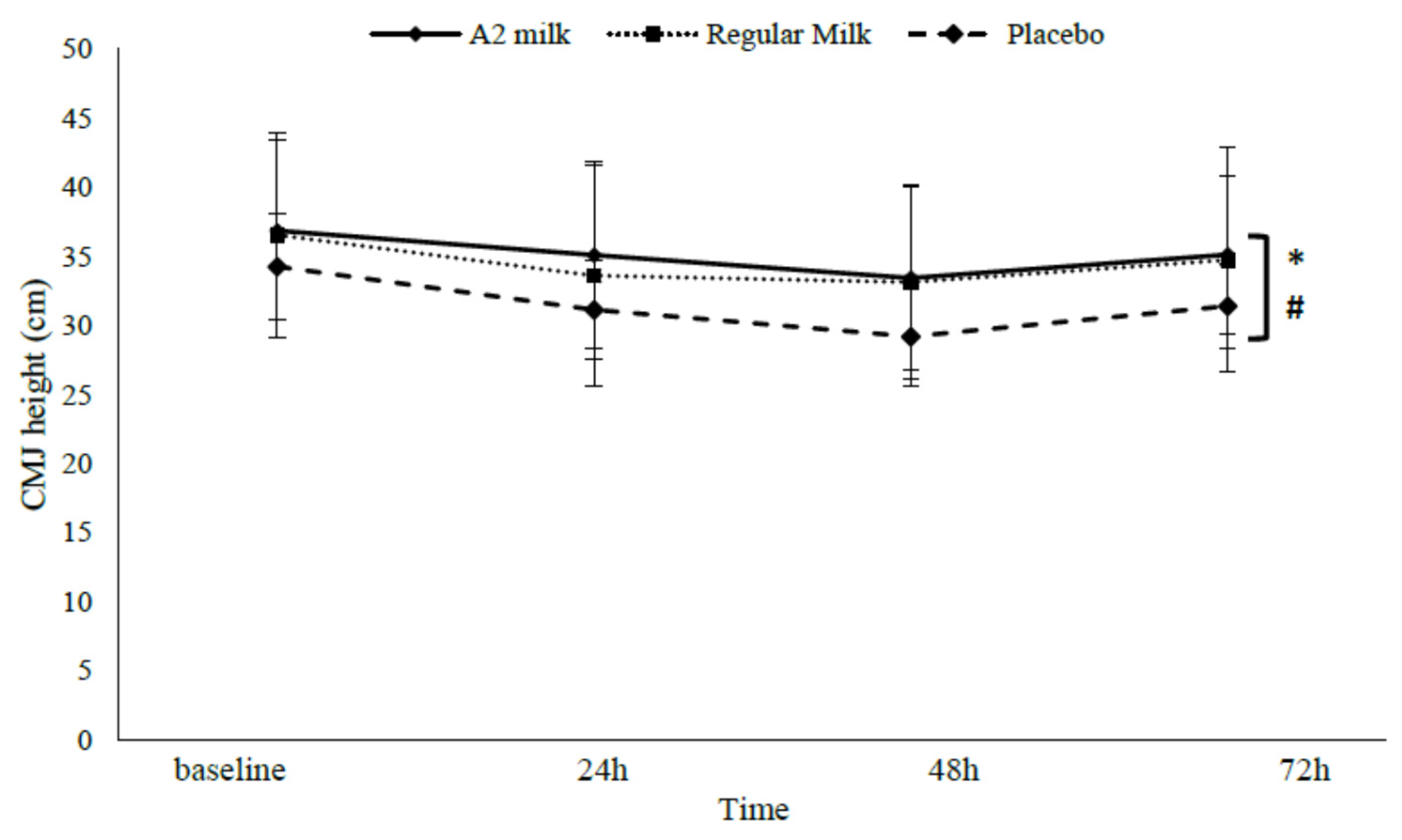
3.3.2. CMJ height
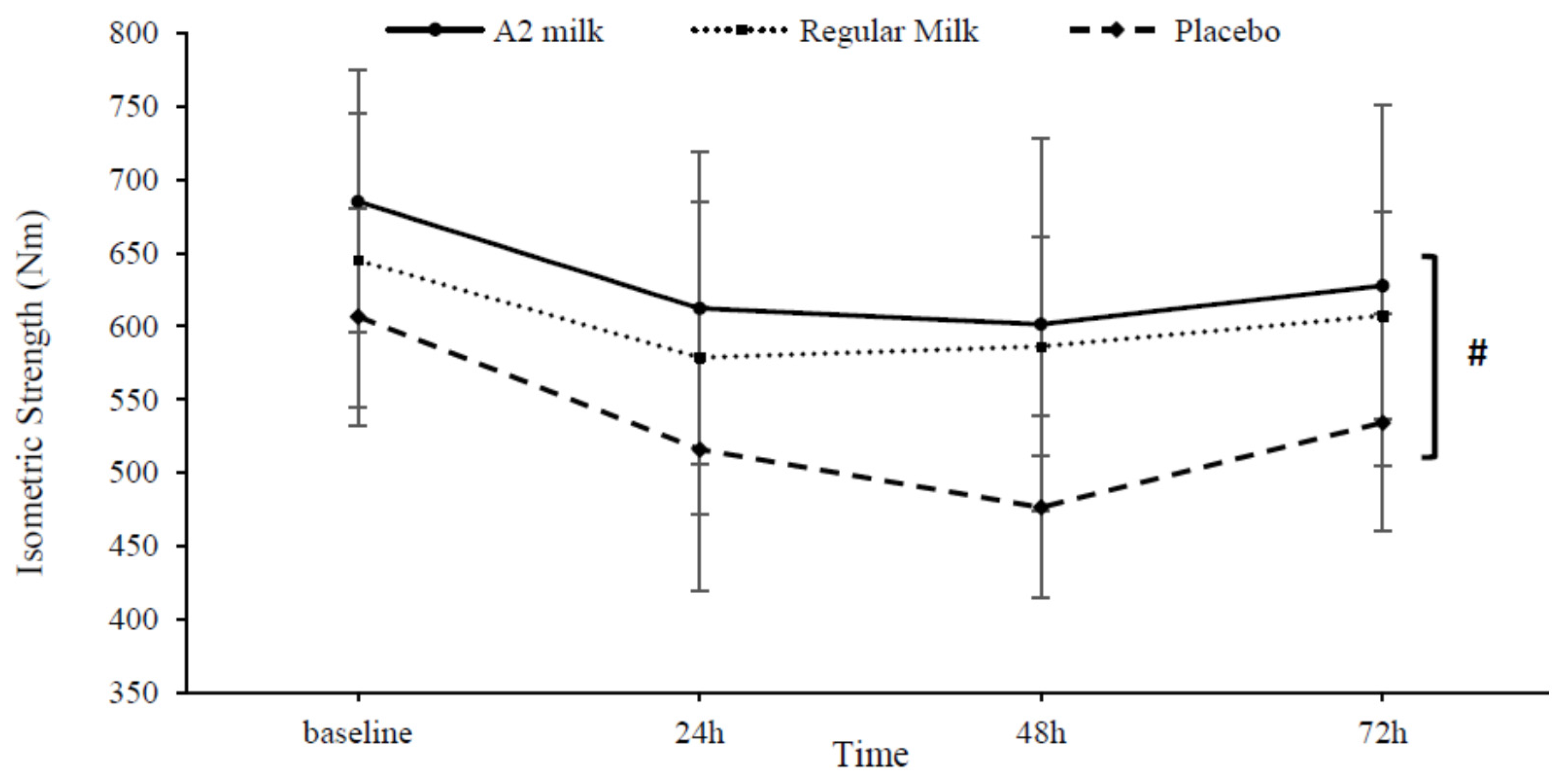
3.3.3. Maximal Voluntary Isometric Contraction (MVIC)
3.3.4. Visual Analogue Scale (Muscle Soreness)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The Effects of Beetroot Juice Supplementation on Indices of Muscle Damage Following Eccentric Exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Shirato, M.; Tsuchiya, Y.; Sato, T.; Hamano, S.; Gushiken, T.; Kimura, N.; Ochi, E. Effects of Combined β-Hydroxy-β-Methylbutyrate (HMB) and Whey Protein Ingestion on Symptoms of Eccentric Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2016, 13, 7. [Google Scholar] [CrossRef]
- Howatson, G.; Milak, A. Exercise-Induced Muscle Damage Following a Bout of Sport Specific Repeated Sprints. J. Strength Cond. Res. 2009, 23, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Eston, R. The Effect of Exercise-Induced Muscle Damage on Isometric and Dynamic Knee Extensor Strength and Vertical Jump Performance. J. Sports Sci. 2002, 20, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gilson, S.F.; Saunders, M.J.; Moran, C.W.; Moore, R.W.; Womack, C.J.; Todd, M.K. Effects of Chocolate Milk Consumption on Markers of Muscle Recovery Following Soccer Training: A Randomized Cross-over Study. J. Int. Soc. Sports Nutr. 2010, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Tee, J.C.; Bosch, A.N.; Lambert, M.I. Metabolic Consequences of Exercise-Induced Muscle Damage. Sports Med. 2007, 37, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The Use of Nonsteroidal Anti-Inflammatory Drugs for Exercise-Induced Muscle Damage. Sports Med. 2012, 42, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Tarnopolsky, M.A.; Yardley, N.J.; Phillips, S.M. Cellular Adaptation to Repeated Eccentric Exercise-Induced Muscle Damage. J. Appl. Physiol. 2001, 91, 1669–1678. [Google Scholar] [PubMed]
- Cobley, J.N.; McGlory, C.; Morton, J.P.; Close, G.L. N-Acetylcysteine’s Attenuation of Fatigue after Repeated Bouts of Intermittent Exercise: Practical Implications for Tournament Situations. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Kruger, M.J.; Smith, R.M.; Myburgh, K.H. The Inflammatory Response to Skeletal Muscle Injury. Sports Med. 2008, 38, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Does Exercise-Induced Muscle Damage Play a Role in Skeletal Muscle Hypertrophy? J. Strength Cond. Res. 2012, 26, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Connolly, D.A.; Eston, R.G.; Gleim, G.W. Exercise-Induced Muscle Damage and Potential Mechanisms for the Repeated Bout Effect. Sports Med. 1999, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Allen, T.J. Damage to Skeletal Muscle from Eccentric Exercise. Exerc. Sport Sci. Rev. 2005, 33, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.N.; Chleboun, G.; Conatser, R. Muscle Stiffness, Strength Loss, Swelling and Soreness Following Exercise-Induced Injury in Humans. J. Physiol. 1993, 464, 183. [Google Scholar] [CrossRef] [PubMed]
- Highton, J.M.; Twist, C.; Eston, R.G. The Effects of Exercise-Induced Muscle Damage on Agility and Sprint Running Performance. J. Exerc. Sci. Fit. 2009, 7, 24–30. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Nosaka, K.; Braun, B. Muscle Function after Exercise-Induced Muscle Damage and Rapid Adaptation. Med. Sci. Sports Exerc. 1992, 24, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Gamble, P. Periodization of Training for Team Sports Athletes. Strength Cond. J. 2006, 28, 56–66. [Google Scholar] [CrossRef]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand Blueberry Consumption on Recovery from Eccentric Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Tranchina, C.P.; Rashti, S.L.; Kang, J.; Faigenbaum, A.D. Effect of a Proprietary Protein Supplement on Recovery Indices Following Resistance Exercise in Strength/Power Athletes. Amino Acids 2010, 38, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular Function after Exercise-Induced Muscle Damage. Sports Med. 2004, 34, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-Induced Muscle Damage is Reduced in Resistance-Trained Males by Branched Chain Amino Acids: A Randomized, Double-Blind, Placebo Controlled Study. J. Int. Soc. Sports Nutr. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The Response of Muscle Protein Synthesis Following Whole-body Resistance Exercise Is Greater Following 40 G than 20 G of Ingested Whey Protein. Physiol. Rep. 2016, 4, e12893. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.J.; Triplett, N.T.; Haines, T.L.; Skinner, J.W.; Fairbrother, K.R.; McBride, J.M. Effect of Leucine Supplementation on Indices of Muscle Damage Following Drop Jumps and Resistance Exercise. Amino Acids 2012, 42, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Bell, P.G.; Stevenson, E. Effect of Milk on Team Sport Performance after Exercise-Induced Muscle Damage. Med. Sci. Sports Exerc. 2013, 45, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Suber, T. Dairy Protein Can Improve the Lives of Billions, Worldwide. Dairy Foods 2015, 116, 22. [Google Scholar]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of Protein Supplements on Muscle Damage, Soreness and Recovery of Muscle Function and Physical Performance: A Systematic Review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of Fluid Skim Milk Promotes Greater Muscle Protein Accretion after Resistance Exercise than Does Consumption of an Isonitrogenous and Isoenergetic Soy-Protein Beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [PubMed]
- Lysenko, E.A.; Vepkhvadze, T.F.; Lednev, E.M.; Vinogradova, O.L.; Popov, D.V. Branched-Chain Amino Acids Administration Suppresses Endurance Exercise-Related Activation of Ubiquitin Proteasome Signaling in Trained Human Skeletal Muscle. J. Physiol. Sci. 2016. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the Effects of Amino Acids and Insulin on Signaling, Ubiquitin Ligases, and Protein Turnover in Human Muscle. AJP Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Holwerda, A.M.; Phillips, S.M.; van Loon, L.J. C. What Is the Optimal Amount of Protein to Support Post-Exercise Skeletal Muscle Reconditioning in the Older Adult? Sports Med. 2016, 46, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.G. Impaired Microvascular Perfusion: A Consequence of Vascular Dysfunction and a Potential Cause of Insulin Resistance in Muscle. AJP Endocrinol. Metab. 2008, 295, E732–E750. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Hayes, P.R.; French, D.N.; Stevenson, E.; St Clair Gibson, A. Acute Milk-Based protein–CHO Supplementation Attenuates Exercise-Induced Muscle Damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Stevenson, E.; Hayes, P.R.; Robson-Ansley, P.; Howatson, G. Effect of Milk-Based Carbohydrate-Protein Supplement Timing on the Attenuation of Exercise-Induced Muscle Damage. Appl. Physiol. Nutr. Metab. 2010, 35, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Robson-Ansley, P.; Hayes, P.R.; Stevenson, E. Effect of Volume of Milk Consumed on the Attenuation of Exercise-Induced Muscle Damage. Eur. J. Appl. Physiol. 2012, 112, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Konopka, A.R.; Jemiolo, B.; Trappe, S.W.; Trappe, T.A.; Reidy, P.T. Muscle Protein Synthesis and Gene Expression during Recovery from Aerobic Exercise in the Fasted and Fed States. AJP Regul. Integr. Comp. Physiol. 2010, 299, R1254–R1262. [Google Scholar] [CrossRef] [PubMed]
- Raastad, T.; Owe, S.G.; Paulsen, G.; Enns, D.; Overgaard, K.; Crameri, R.; Kiil, S.; Belcastro, A.; Bergersen, L.; HalléN, J. Changes in Calpain Activity, Muscle Structure, and Function after Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rankin, P.; Stevenson, E.; Cockburn, E. The Effect of Milk on the Attenuation of Exercise-Induced Muscle Damage in Males and Females. Eur. J. Appl. Physiol. 2015, 115, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Bonci, L. Supplements: Help, Harm, or Hype? How to Approach Athletes. Curr. Sports Med. Rep. 2009, 8, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.D. Milk: The New Sports Drink? A Review. J. Int. Soc. Sports Nutr. 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Aparici, A.; Pérez, M.J.; Rodríguez, P. Perception of Lactose Intolerance Impairs Health-Related Quality of Life. Eur. J. Clin. Nutr. 2016, 70, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Woodford, K.B. Devil in the Milk: Illness, Health and Politics of A1 and A2 Milk; Chelsea Green Publishing: Vermont, VT, USA, 2009. [Google Scholar]
- Suchy, F.J.; Brannon, P.M.; Carpenter, T.O.; Fernandez, J.R.; Gilsanz, V.; Gould, J.B.; Hall, K.; Hui, S.L.; Lupton, J.; Mennella, J.; et al. NIH Consensus Development Conference Statement: Lactose Intolerance and Health. NIH Consens. State Sci. Statements 2010, 27, 1–27. [Google Scholar] [PubMed]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of Milk Containing Only A2 Beta Casein versus Milk Containing Both A1 and A2 Beta Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative Effects of A1 versus A2 Beta-Casein on Gastrointestinal Measures: A Blinded Randomised Cross-over Pilot Study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.P. G.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 β-Casein Affects Gastrointestinal Transit Time, Dipeptidyl Peptidase-4 Activity, and Inflammatory Status Relative to A2 β-Casein in Wistar Rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A.S. The A2 Milk Case: A Critical Review. Eur. J. Clin. Nutr. 2005, 59, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Hammouda, O.; Chaouachi, A.; Chamari, K.; Souissi, N. The Effect of Time-of-Day and Ramadan Fasting on Anaerobic Performances. Int. J. Sports Med. 2012, 33, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Salicki, R.; Goodall, S.; Thomas, K.; Howatson, G. Muscle Damage Response in Female Collegiate Athletes after Repeated Sprint Activity. J. Strength Cond. Res. 2015, 29, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Winchester, J.B.; Nelson, A.G.; Landin, D.; Young, M.A.; Schexnayder, I.C. Static Stretching Impairs Sprint Performance in Collegiate Track and Field Athletes. J. Strength Cond. Res. 2008, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Wardle, S.L.; Macnaughton, L.S.; Hodgson, A.B.; Tipton, K.D. Protein Considerations for Optimising Skeletal Muscle Mass in Healthy Young and Older Adults. Nutrients 2016, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Aoi, W.; Mune, K.; Yamauchi, H.; Furuta, K.; Sasaki, S.; Takeda, K.; Harada, K.; Wada, S.; Nakamura, Y.; et al. Fermented Milk Improves Glucose Metabolism in Exercise-Induced Muscle Damage in Young Healthy Men. Nutr. J. 2013, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Gaeini, A.A.; Kordi, M.R.; Rahimi, M.; Rahnama, N.; Bambaeichi, E. Effect of Various Ratios of Carbohydrate-Protein Supplementation on Resistance Exercise-Induced Muscle Damage. J. Sports Med. Phys. Fit. 2012, 52, 151–157. [Google Scholar]
- Baty, J.J.; Hwang, H.; Ding, Z.; Bernard, J.R.; Wang, B.; Kwon, B.; Ivy, J.L. The Effect of a Carbohydrate and Protein Supplement on Resistance Exercise Performance, Hormonal Response, and Muscle Damage. J. Strength Cond. Res. 2007, 21, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilkinson, S.B. Impact of Resistance and Endurance Exercise and Ingestion of Varying Protein Sources on Changes in Human Skeletal Muscle Protein Turnover. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2015. [Google Scholar]
- McGlory, C.; Phillips, S.M. Exercise and the Regulation of Skeletal Muscle Hypertrophy. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 135, pp. 153–173. [Google Scholar]
- Phillips, S.M.; van Loon, L.J. Dietary Protein for Athletes: From Requirements to Optimum Adaptation. J. Sports Sci. 2011, 29 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Guillet, C.; Garcia-Rodenas, C.; Gachon, P.; Bouteloup-Demange, C.; Reiffers-Magnani, K.; Fauquant, J.; Ballèvre, O.; Beaufrère, B. The Rate of Protein Digestion Affects Protein Gain Differently during Aging in Humans. J. Physiol. 2003, 549, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, Cytokines and Satellite Cells: What Role Do They Play in Muscle Damage and Regeneration Following Eccentric Exercise. Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar] [PubMed]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with Casein or Total Milk Protein, Digestion of Milk Soluble Proteins Is Too Rapid to Sustain the Anabolic Postprandial Amino Acid Requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [PubMed]
- Malm, C.; Nyberg, P.; Engström, M.; Sjödin, B.; Lenkei, R.; Ekblom, B.; Lundberg, I. Immunological Changes in Human Skeletal Muscle and Blood after Eccentric Exercise and Multiple Biopsies. J. Physiol. 2000, 529, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Friden, J.; Sjöström, M.; Ekblom, B. Myofibrillar Damage Following Intense Eccentric Exercise in Man. Int. J. Sports Med. 1983, 4, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed Muscle Protein Synthesis and Breakdown after Resistance Exercise in Humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E99–E107. [Google Scholar]
- Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Bellar, D.; LeBlanc, N.R.; Murphy, K.; Moody, K.M.; Buquet, G. The Impact of Chocolate Goat’s and Cow’s Milk on Postresistance Exercise Endocrine Responses and Isometric Mid-Thigh Pull Performance. J. Diet. Suppl. 2016, 13, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar Muscle Protein Synthesis Rates Subsequent to a Meal in Response to Increasing Doses of Whey Protein at Rest and after Resistance Exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K.; et al. Branched-Chain Amino Acid Supplementation before Squat Exercise and Delayed-Onset Muscle Soreness. Int. J. Sport Nutr. 2010, 20, 236. [Google Scholar] [CrossRef]
- Hatchett, A.; Berry, C.; Oliva, C.; Wiley, D.; St. Hilaire, J.; LaRochelle, A. A Comparison between Chocolate Milk and a Raw Milk Honey Solution’s Influence on Delayed Onset of Muscle Soreness. Sports 2016, 4, 18. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]


| Group | Baseline MVIC (N·m) | Age (Years) | Height (m) | Weight (kg) |
|---|---|---|---|---|
| A2 milk | 685 ± 89 | 23 ± 3 | 178.8 ± 5.1 | 79.4 ± 10.1 |
| Regular Milk | 644 ± 74 | 23 ± 1 | 183.0 ± 8.6 | 81.4 ± 13.1 |
| Placebo | 606 ± 74 | 22 ± 1 | 180.7 ± 5.5 | 77.1 ± 7.8 |
| Group | Energy | PRO | CHO | Fat |
|---|---|---|---|---|
| A2 milk | 1005 kJ/235 kcal | 18 g | 24 g | 9 g |
| Regular milk | 1046 kJ/250 kcal | 18 g | 24 g | 9 g |
| Placebo | 920.5 kJ/220 kcal | 0 g | 50 g | 0 g |
| Group | Energy | PRO | CHO | Fat |
|---|---|---|---|---|
| A2 milk | 11,984 kcal | 510 g | 1604 g | 452 g |
| Regular milk | 11,879 kcal | 571 g | 1390 g | 419 g |
| Placebo | 11,451 kcal | 584 g | 1403 g | 420 g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirk, B.; Mitchell, J.; Jackson, M.; Amirabdollahian, F.; Alizadehkhaiyat, O.; Clifford, T. A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes? Nutrients 2017, 9, 94. https://doi.org/10.3390/nu9020094
Kirk B, Mitchell J, Jackson M, Amirabdollahian F, Alizadehkhaiyat O, Clifford T. A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes? Nutrients. 2017; 9(2):94. https://doi.org/10.3390/nu9020094
Chicago/Turabian StyleKirk, Ben, Jade Mitchell, Matthew Jackson, Farzad Amirabdollahian, Omid Alizadehkhaiyat, and Tom Clifford. 2017. "A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes?" Nutrients 9, no. 2: 94. https://doi.org/10.3390/nu9020094
APA StyleKirk, B., Mitchell, J., Jackson, M., Amirabdollahian, F., Alizadehkhaiyat, O., & Clifford, T. (2017). A2 Milk Enhances Dynamic Muscle Function Following Repeated Sprint Exercise, a Possible Ergogenic Aid for A1-Protein Intolerant Athletes? Nutrients, 9(2), 94. https://doi.org/10.3390/nu9020094






