Dietary Animal Plasma Proteins Improve the Intestinal Immune Response in Senescent Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Design
2.3. MLN Cell Isolation
2.4. PP Cell Isolation
2.5. Cell Staining
2.6. Serum Cytokine Determination
2.7. Real-Time Polimerase Chain Reaction
2.8. Statistical Analysis
3. Results
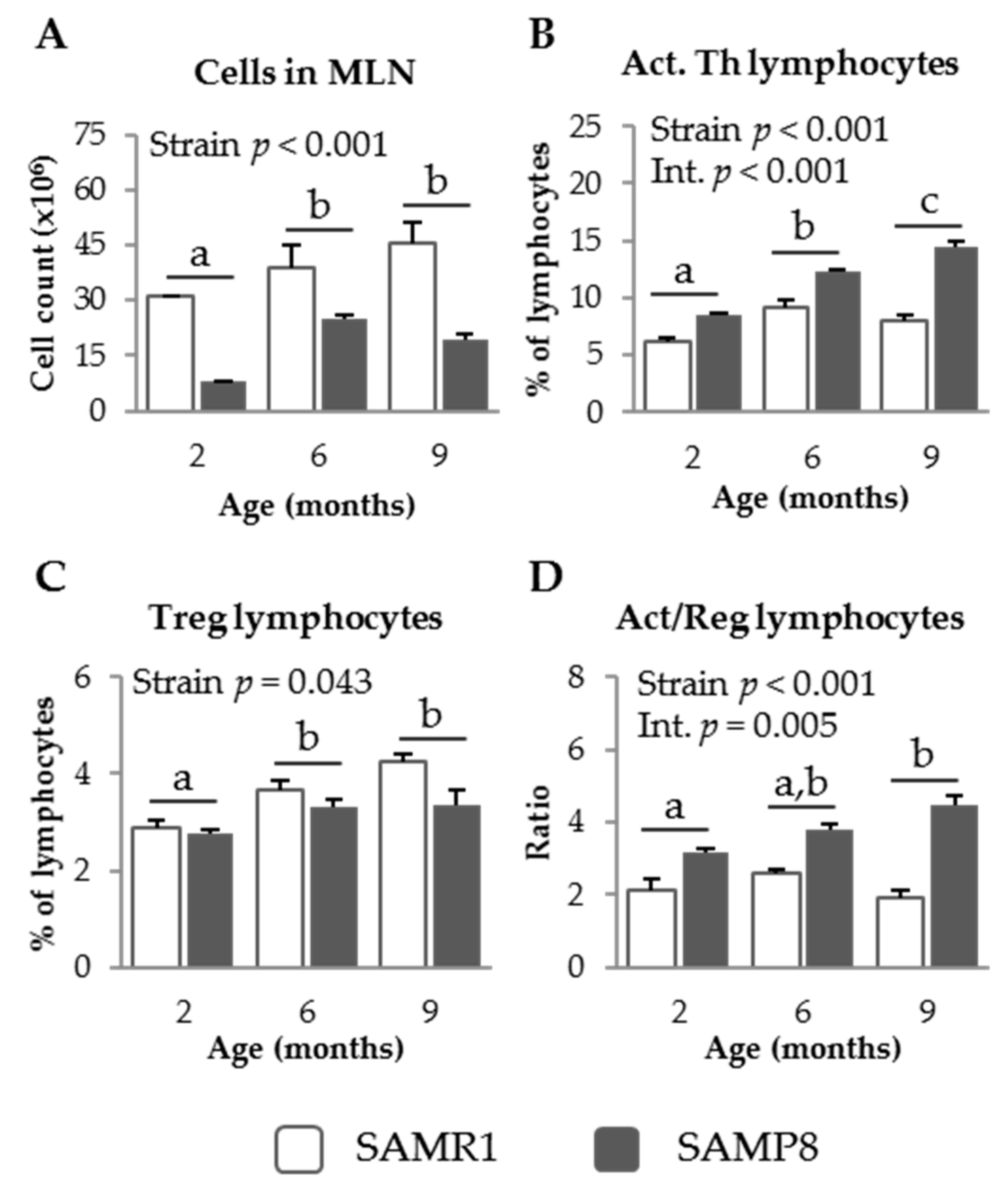
3.1. Characterization of GALT in SAM Strains
3.1.1. MLN Populations in SAM Strains
3.1.2. PP Populations in SAM Strains
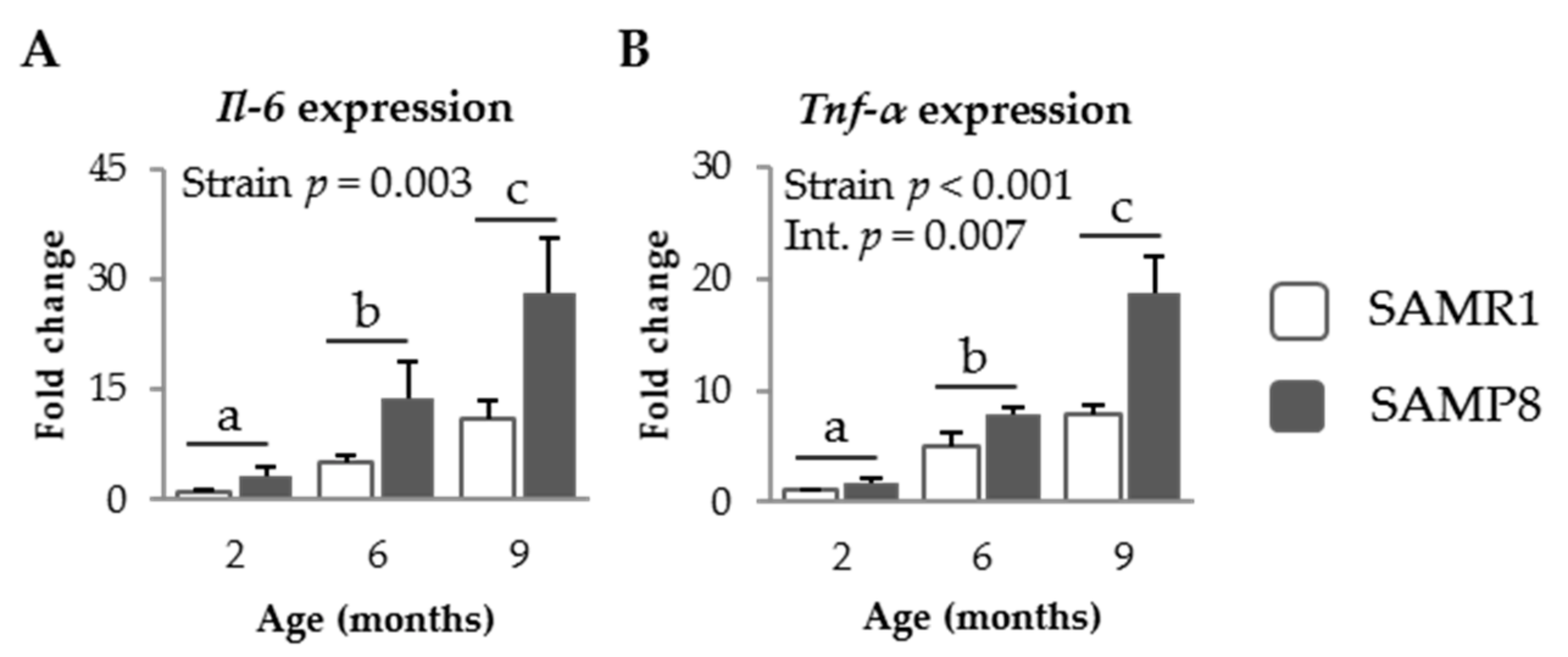
3.1.3. Pro-Inflammatory Cytokines in SAM Strains
3.2. Effect of SDP on Senescent Mice
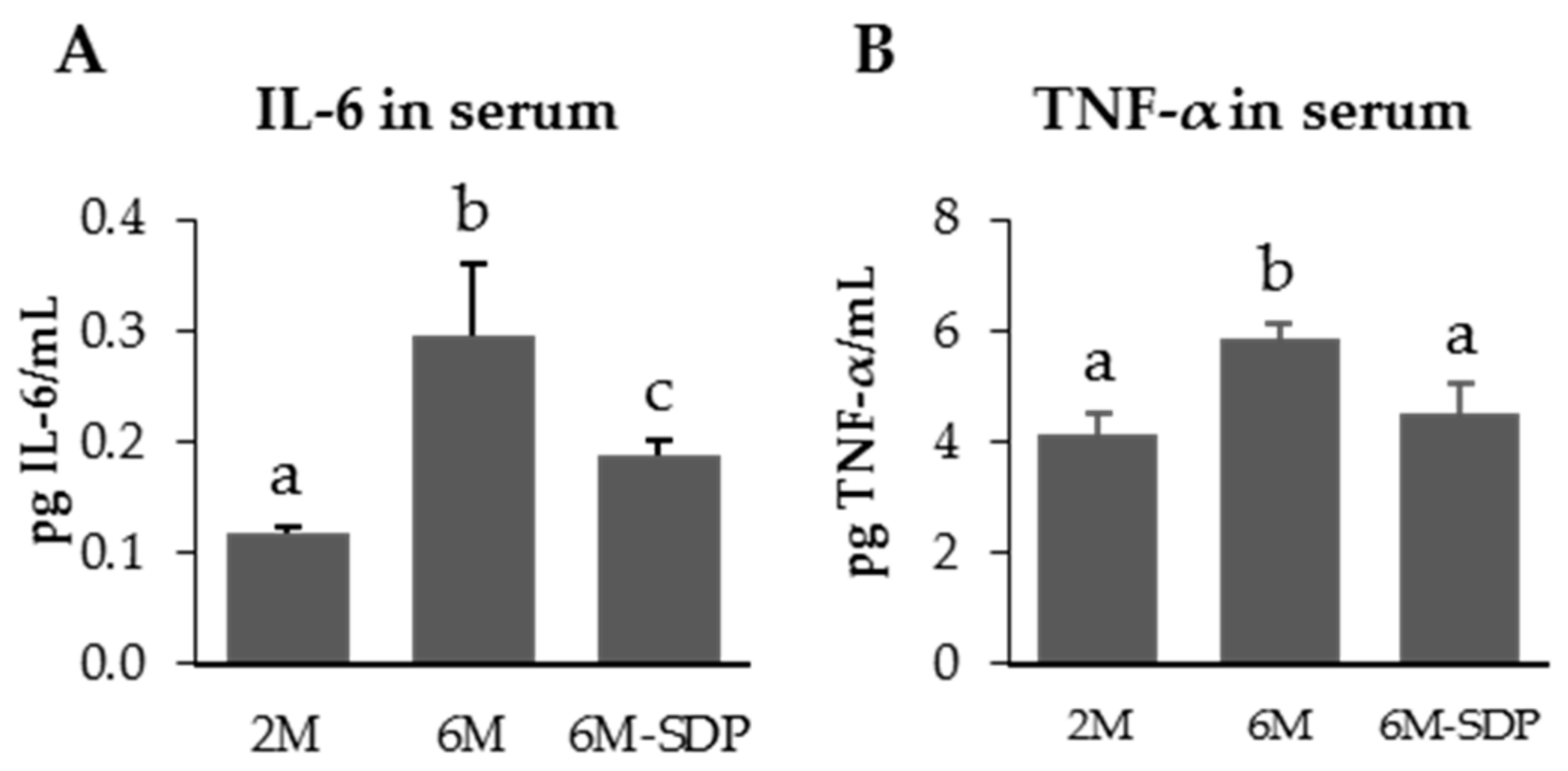
3.2.1. Effect of SDP on Systemic Variables
3.2.2. Effect of SDP on MLN Populations in Senescent Mice
3.2.3. Effect of SDP on PP Populations in Senescent Mice
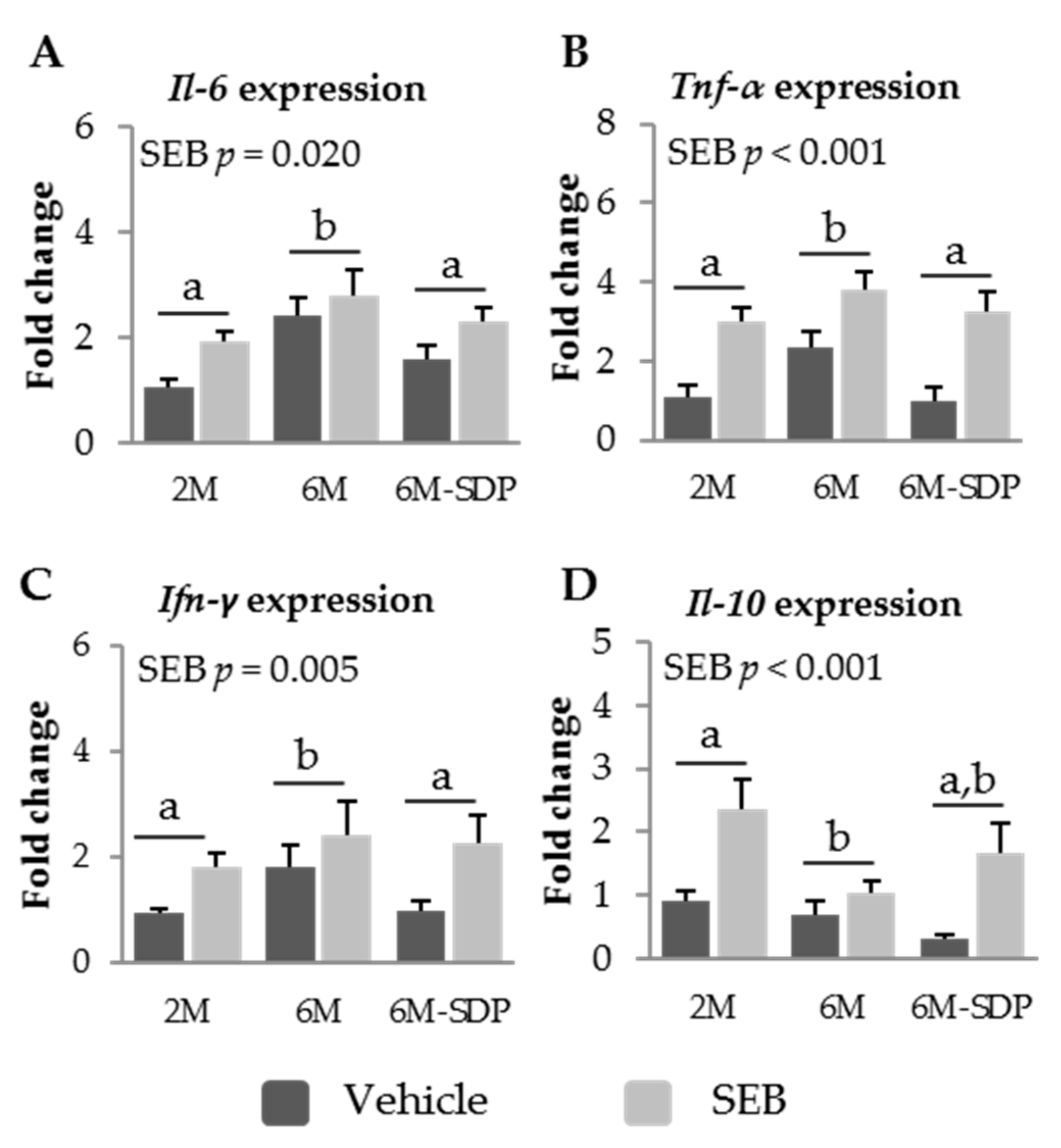
3.2.4. Effect of SDP on Cytokine Expression in Senescent Mice
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 2, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, S.; Ponnappan, U. Aging and immune function: Molecular mechanisms to interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef] [PubMed]
- Brüünsgaard, H.; Pedersen, B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. N. Am. 2003, 23, 15–39. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevenini, E.; Monti, D.; Franceschi, C. Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.D.; Acharya, K.R. Superantigens: Structure-function relationships. Int. J. Med. Microbiol. 2004, 293, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Pelegrí, C.; Vicario, M.; Castell, M.; Russell, L.; Campbell, J.M.; Quigley, J.D., III; Polo, J.; Amat, C.; Moretó, M. Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus Superantigen B. J. Nutr. 2004, 134, 2667–2672. [Google Scholar]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with Staphylococcus aureus enterotoxin B. J. Nutr. 2008, 138, 533–537. [Google Scholar] [PubMed]
- Pérez-Bosque, A.; Miró, L.; Amat, C.; Polo, J.; Moretó, M. The anti-inflammatory effect of spray-dried plasma is mediated by a reduction in mucosal lymphocyte activation and infiltration in a mouse model of intestinal inflammation. Nutrients 2016, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bosque, A.; Amat, C.; Polo, J.; Campbell, J.M.; Crenshaw, J.; Russell, L.; Moretó, M. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J. Nutr. 2006, 136, 2838–2843. [Google Scholar] [PubMed]
- Pérez-Bosque, A.; Miró, L.; Polo, J.; Russell, L.; Campbell, J.; Weaver, E.; Crenshaw, J.; Moretó, M. Dietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in rats. J. Nutr. 2010, 140, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T. Senescence-accelerated mouse (SAM): A biogerontological resource in aging research. Neurobiol. Aging 1999, 20, 105–110. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Pawelec, G.; Wong, S.C.; Goldeck, D.; Tai, J.J.Y.; Fulop, T. Impact of age on T cell signaling: A general defect or specific alterations? Ageing Res. Rev. 2011, 10, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Franceschi, C.; Mazzatti, D.; Solana, R.; Wikby, A.; Pawelec, G. Aging of the immune system as a prognostic factor for human longevity. Physiology 2008, 23, 64–74. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.G.; Leach, M.R.; Huang, C.; Wang, C.; Newberry, R.D. Aging impacts isolated lymphoid follicle development and function. Immun. Ageing 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Hosokawa, M.; Higuchi, K. Senescence-accelerated mouse (SAM): A murine model of accelerated senescence. J. Am. Geriatr. Soc. 1991, 39, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Yoshida, H.; Doi, T.; Muso, E.; Ohshio, G.; Higuchi, K.; Inada, M.; Miyake, T.; Kita, T.; Hamashima, Y.; et al. Autoimmune abnormalities in a murine model of accelerated senescence. Clin. Exp. Immunol. 1989, 75, 129–135. [Google Scholar] [PubMed]
- McNerlan, S.E.; Rea, I.M.; Alexander, H.D. A whole blood method for measurement of intracellular TNF-alpha, IFN-gamma and IL-2 expression in stimulated CD3+ lymphocytes: Differences between young and elderly subjects. Exp. Gerontol. 2002, 37, 227–234. [Google Scholar] [CrossRef]
- Ershler, W.B.; Keller, E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.; Kireev, R.; Forman, K.; García, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8). Exp. Gerontol. 2010, 45, 950–956. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005, 579, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, L.; Wang, H.; Ma, H.; Liu, G.; Zhao, Y. Changes of CD4+ CD25+ Foxp3+ regulatory T cells in aged Balb/c mice. J. Leukoc. Biol. 2007, 81, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hurez, V.J.; Thibodeaux, S.R.; Kious, M.J.; Liu, A.; Lin, P.; Murthy, K.; Pandeswara, S.; Shin, T.; Curiel, T.J. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012, 11, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional regulatory T cells accumulate in aged hosts and promote chronic in factious disease reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Onai, H.; Kudo, S. Suppression of superantigen-induced lung injury and vasculitis by preadministration of human urinary trypsin inhibitor. Eur. J. Clin. Investig. 2001, 31, 272–280. [Google Scholar] [CrossRef]
- Olivieri, D.; Chetta, A. Therapeutic perspectives in vascular remodeling in asthma and chronic obstructive pulmonary disease. Chem. Immunol. Allergy 2013, 99, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Nacionales, D.C.; Gentile, L.F.; Vanzant, E.; Lopez, M.C.; Cuenca, A.; Cuenca, A.G.; Ungaro, R.; Li, Y.; Baslanti, T.O.; Bihorac, A.; et al. Aged mice are unable to mount an effective myeloid response to sepsis. J. Immunol. 2014, 192, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Palmer, J.L.; Plackett, T.P.; Deburghgraeve, C.R.; Kovacs, E.J. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp. Gerontol. 2014, 54, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Torrallardona, D.; Conde, M.R.; Badiola, I.; Polo, J.; Brufau, J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J. Anim. Sci. 2003, 81, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Martín-Orúe, S.M.; Pérez-bosque, A.; Gómez de Segura, A.; Moretó, M. Feed added spray-dried porcine plasma modifies cecal microbiota in rats. In Proceedings of the Gut Microbiome Meeting, Clermont-Ferrand, France, 18 June 2008. [Google Scholar]
- Moretó, M.; Miró, L.; Garcia-Just, A.; Amat, C.; Pérez-Bosque, A. La suplementación dietética con proteínas de plasma porcino modifica la composición de la microbiota fecal de ratones. In Proceedings of the XXXVIII Congreso de la Sociedad Española de Bioquímica y Biología Molecular, València, Spain, 7 September 2015; Rubes Editorial, S.L.: Barcelona, Spain, 2015; p. 78. [Google Scholar]
- Moretó, M.; Miró, L.; Amat, C.; Polo, J.; Pérez-Bosque, A. Dietary supplementation with spray-dried animal plasma proteins modifies the profile of the fecal microbiota in young mice. In Proceedings of the 5th World Congress on Targeting Microbiota, Berlin, Germany, 26 October 2017. [Google Scholar]




| Ingredients | Control Diet | SDP 1 Diet |
|---|---|---|
| g/kg | ||
| SDP | - | 80.0 |
| Dried skim milk | 530.7 | 340.5 |
| Corn starch | 199.3 | 308.8 |
| Sucrose | 94.5 | 94.5 |
| Soybean oil | 70 | 70 |
| Cellulose | 50 | 50 |
| AIN-93-G-MX (94046) 2 | 35 | 35 |
| AIN-93 VX (94047) 2 | 15 | 15 |
| DL-Methionine | 2.5 | 3.2 |
| Choline bitartrate | 3 | 3 |
| Primer | Forward (5′-3′) | Reverse (5′-3′) | Fragment Size | Accession Number |
|---|---|---|---|---|
| Gusβ | CCGATTATCCAGAGCGAGTATG | CTCAGCGGTGACTGGTTCG | 197 bp | NM_010368.1 |
| Il-6 | ACCAGAGGAAATTTTCAATAGGC | TGATGCACTTGCAGAAAACA | 109 bp | NM_031168.1 |
| Il-10 | GGCGCTGTCATCGATTTCTCCCC | TGGCCTTGTAGACACCTTGGTCTT | 102 bp | NM_010548.2 |
| Inf-γ | CCTTCTTCAGCAACAGCAAGGCG | CTTGGCGCTGGACCTGTGGG | 87 bp | NM_008337.4 |
| Tnf-α | CCACCACGCTCTTCTGTCTAC | AGGGTCTGGGCCATAGAACT | 103 bp | NM_013693.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miró, L.; Garcia-Just, A.; Amat, C.; Polo, J.; Moretó, M.; Pérez-Bosque, A. Dietary Animal Plasma Proteins Improve the Intestinal Immune Response in Senescent Mice. Nutrients 2017, 9, 1346. https://doi.org/10.3390/nu9121346
Miró L, Garcia-Just A, Amat C, Polo J, Moretó M, Pérez-Bosque A. Dietary Animal Plasma Proteins Improve the Intestinal Immune Response in Senescent Mice. Nutrients. 2017; 9(12):1346. https://doi.org/10.3390/nu9121346
Chicago/Turabian StyleMiró, Lluïsa, Alba Garcia-Just, Concepció Amat, Javier Polo, Miquel Moretó, and Anna Pérez-Bosque. 2017. "Dietary Animal Plasma Proteins Improve the Intestinal Immune Response in Senescent Mice" Nutrients 9, no. 12: 1346. https://doi.org/10.3390/nu9121346




