1. Introduction
During the climacteric period, many women between the ages of 40 and 65 years experience and complain of uncomfortable symptoms such as vasomotor symptoms, night sweats, cognitive impairment, insomnia, depression, and irritability [
1,
2]. In addition, body weight gain, fatigue and hot flashes are major symptoms characterized of menopause women. Many symptoms of menopause were closely associated with level of estrogen. These postmenopausal conditions are related to an increased risk of metabolic diseases, such as obesity, heart disease, diabetes, and hypertension, due to estrogen deficiency [
3,
4]. Additionally, estrogen deficiency is recognized as a common risk factor for osteoporosis. Estrogen deficiency is related to an atherogenic lipid profile, characterized by high density lipoprotein (HDL)-cholesterol, low density lipoprotein (LDL)-cholesterol, triglyceride levels [
5], central adiposity [
6], increased diastolic pressure [
7], and increased insulin resistance [
8]. Thus, for attenuating estrogen deficiency of menopausal symptoms, synthetic estrogens were administered to women during menopause. These hormones are as protective as estrogens in the reduction of symptoms [
9,
10,
11].
As mentioned above, hormone therapy has been used to protect women against climacteric symptoms; however, long-term exposure can cause cardiovascular events and breast cancer [
9,
10,
11]. Thus, phytosubstances from plants, as an alternative to hormone therapy, have attracted much attention [
12,
13,
14]. They have similar structures to estrogens and can bind to estrogen receptors, and therefore were named phytoestrogens (PEs). Several PEs, plant-derived chemicals, act as free radical scavengers. Isoflavones, derived from soy and soy derivatives, are representative PEs. Genistein and daidzein are the most abundant and well studied. This class of PEs can also be found in clover. Purified phytohormones show improved activity in the body and enhanced bioavailability [
12]. PEs can bind to estrogen receptors due to the presence of a phenolic ring and function like estrogens [
12,
13,
15]. Coumestrol and the isoflavonoids genistein, daidzein, and their plant precursors are found mainly in soybeans and clover [
16]. Isoflavones, especially those derived from plants, have various biological activities, can improve metabolic symptoms [
17], and have bone-protective effects [
18] during menopause. Recently, it has been suggested that various pharmacological effects of plant extracts can be enhanced synergistically by appropriate mixed formulations [
19,
20,
21].
Red clover (RC,
Trifolium pratense L.) shows estrogenic effects due to isoflavones and, to a lesser extent, coumestans [
22]. Thus, RC botanical dietary supplements have been used for the treatment of menopausal symptoms, maintenance/improvement of cardiovascular health, and their reported benign effects on the breast, endometrium, and neural structures [
23]. The isoflavonoids formononetin, biochanin A, genistein, and daidzain are present in RC as glycosides and malonates [
24].
Pomegranates (
Punica granatum L.) contain various flavonoids and anthocyanidins, and their main active substances are polyphenols, which show antioxidative, antimutagenic, antiinflammatory, and antimicrobial activities [
25]. The phytoestrogenic effects of pomegranate [
26] are due mainly to isoflavonoids via antioxidant and antiinflammatory pathways [
15,
27]. Recently, pomegranate extract has been shown to be a selective estrogen receptor modulator [
28].
Based on the information above, we hypothesized that dried pomegranate concentrate powder (PCP) might potentiate the anti-climacteric effects of RC. Appropriate mixed formulations of RC and PCP may be expected to show more favorable synergistic anti-climacteric effects. Thus, this study aimed to ascertain the optimal range of RC + PCP to induce anti-climacteric effects. Additionally, we examined whether the anti-climacteric activity of RC could be enhanced by the addition of PCP in ovariectomized (OVX) mice.
2. Experimental Section
2.1. Animals and Husbandry
Kwl:ddY mice (virgin female specific pathogen-free outbred mice) (6 weeks old upon receipt) (Kiwa, Wakayama, Japan) were selected follow to the acclimatization for 16 days. The animals (4 mice per polycarbonate cage) were maintained with a controlled temperature (20–25 °C) and humidity (45%–55%) under 12-h:12-h light:dark cycles. Tap water and normal rodents pellet diet (
Table 1; 38057; Purinafeed, Seungnam, Korea) were given ad libitum. All mice were placed in individual cages containing 150 g of diet and 250 mL of water and the remaining amounts were measured at 24 h after feed supply using an automatic electronic balance (Precisa Instruments) and a measuring cylinder (Pyrex, Corning, NY, USA), respectively. This was regarded as the individual daily food (g/24 h/mouse) and water (mL/24 h/mouse) rations [
29]. All laboratory animals were controlled according to national regulations for the usage and welfare of laboratory animals and approved by the Institutional Animal Care and Use Committee of Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) prior to the experiments (Approval No. DHU2014-020). In addition, experiments on osteoporosis were performed based on United States Food and Drug Administration guidelines [
30].
2.2. Experimental Groups
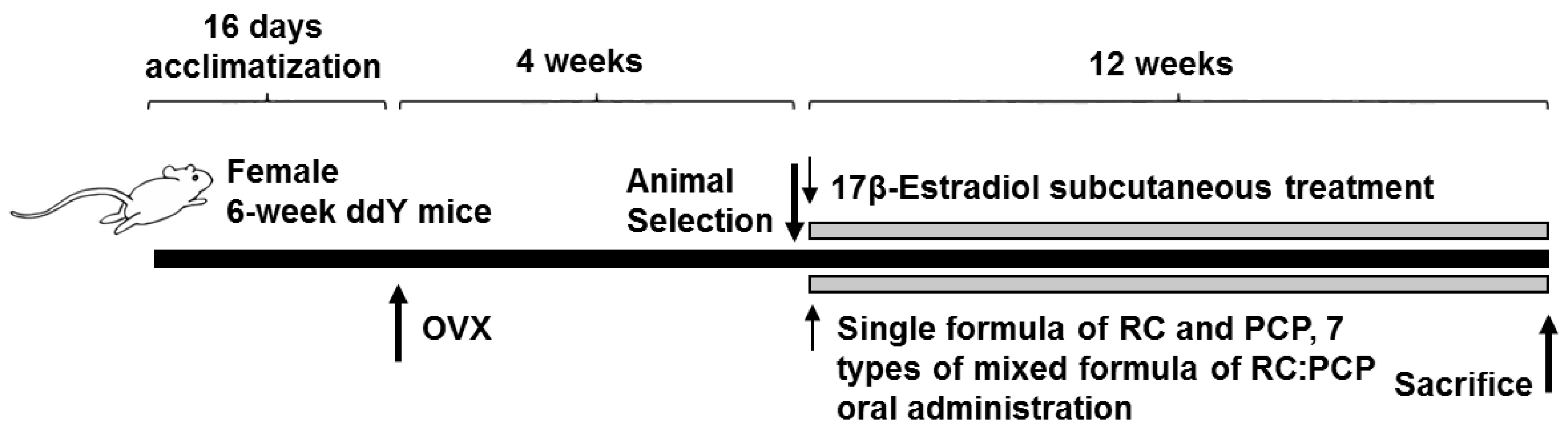
The dose level of 120 mg/kg was selected as dosages of mixed formula consisted of RC:PCP on the clinical dosage in human (mouse dosage = about 12-fold that of human dosage; (600 mg/60 kg) × 12 = 120 mg/kg). The experimental groups were divided into the following 12 groups (8 mice per group): sham vehicle control (sham control); OVX-operated (OVX control); OVX-operated mice + 17β-estradiol (0.03 μg/head); OVX-operated mice + 120 mg/kg RC; OVX-operated mice + 120 mg/kg PCP; OVX-operated mice + 120 mg/kg RC:PCP 1:1 (60:60 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 1:2 (40:80 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 1:4 (24:96 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 1:8 (13:107 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 2:1 (80:40 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 4:1 (96:24 mg/kg) mixture; OVX-operated mice + 120 mg/kg RC:PCP 8:1 (107:13 mg/kg) (g/g) mixture.
2.3. Experimental Design
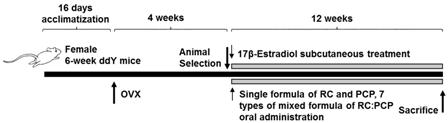
In the present study, Kwl:ddY mice (6 weeks old upon receipt) were prepared and ovariectomy was performed 16 days after acclimatization. Mice were anesthetized with an intraperitoneal injection of 25 mg/kg of Zoletile (Zoletile 50™; Virbac Laboratories, Carros, France) and maintained with 1%–1.5% isoflurane (Hana Pharmaceutical Co., Hwasung, Korea) in a mixture of 70% N
2O and 28.5% O
2. Surgery was conducted according to established methods [
31]. The second group of mice underwent a sham operation in which a similar incision in the linea alba was made but bilateral ovariectomy was not performed.
At 28 days after surgery, 8 mice per group were selected based on body weight and RC (120 mg/kg), PCP (120 mg/kg), or an RC:PCP mixture (g/g) was administered orally once a day for 84 days. Standardized RC, PCP, and RC:PCP 1:1, 1:2, 1:4, 1:8, 2:1, 4:1, and 8:1 (g/g) mixture were provided by HEALTH-LOVE Co., Ltd. (Anyang, Korea). The RC substance contained 8% total isoflavones, 0.62% genistein, 5.43% biochanin A, 3.66% formononetin, and 0.47% daidzein suspended in 12 mg/mL of distilled water. The PCP substance contained 0.90 mg/g of ellagic acid dissolved in 12 mg/mL of distilled water. Appropriate amounts of RC, PCP, and the RC:PCP mixture (g/g) were directly suspended or dissolved in distilled water and administered in a volume of 10 mL/kg. In OVX and sham control mice, only distilled water was administered orally as a vehicle in equal volumes and periods instead of the herbal formulas. In addition, 17β-estradiol (Sigma-Aldrich, St. Louis, MO, USA) 0.03 μg was dissolved in 0.2 mL of sterilized saline, and subcutaneously treated on the dorsal back skins in a volume of 0.2 mL/mouse (0.03 μg/head/day). After 84 days of continuous oral administration, the mice were anesthetized with 50 mg/kg of Zoletile and dissected according to established methods [
32] (
Figure 1).
Differences in body weight were measured at the time of ovariectomy, 1 day before RC, PCP, and RC:PCP administration, and once a week from the initiation of administration to termination using an automatic electronic balance (Precisa Instruments, Dietikon, Switzerland) [
29]. At ovariectomy, the first administration, and at termination, food, but not water, was withheld from the experimental animals (approximately 18 h prior) to reduce differences due to feeding.
2.4. Measurement of the Bone Mineral Density (BMD) and Body Fat Density
The mean BMD of total body and the right femur were determined using in live dual-energy X-ray absorptionmetry (DEXA; InAlyzer, Medikors, Seungnam, Korea). In addition, mean fat densities on the body and abdominal cavity regions of each mouse, respectively.
2.5. Organ Weight Measurements
At sacrifice, the abdominal fat pads deposited in the abdominal cavity, total liver, and uterus (including vagina) were collected after removing the surrounding connective tissues, muscles, and any debris, after which the weights of organs were measured at g levels for absolute wet-weights. To reduce the individual body weight differences, the relative weights (% of body weight) were calculated at sacrifice.
2.6. Bone Weight Measurements
At sacrifice, the right sides of the femurs were collected after removing the surrounding connective tissues, muscles, and any debris. The bone weight was measured at g levels regarding absolute wet-weights, and they were dried at 120 °C for 8 h in a high temperature dry oven (LDO-080N, Daihan Labtech Co., Seoul, Korea) for measurements of dry bone weights. Next, dried bones were carbonized at 800 °C for 6 h in a furnace (LEF-1055-1, Daihan Labtech Co.) to measure ash absolute weights. To reduce the individual body weight differences, the relative weight (%) was calculated the proportion of absolute wet/dry/ash weight based on the body weight at sacrifice.
2.7. Measurement of Bone Strengths
Bone strength was detected as failure load (FL). FL of mid-shaft regions of right femurs were detected using a three-point bending test to failure using a computerized testing machine (SV-H1000, Japan Instrumentation System Co., Tokyo, Japan) as N (Newton).
2.8. Blood Collection
For serum biochemistry, approximately 1 mL of whole blood was collected from vena cava at sacrifice and separated from the serum by centrifugation at 21,000× g for 10 min at 4 °C using a clotting activated serum tube. All serum samples were frozen at −150 °C until they were assayed.
2.9. Serum Biochemistry
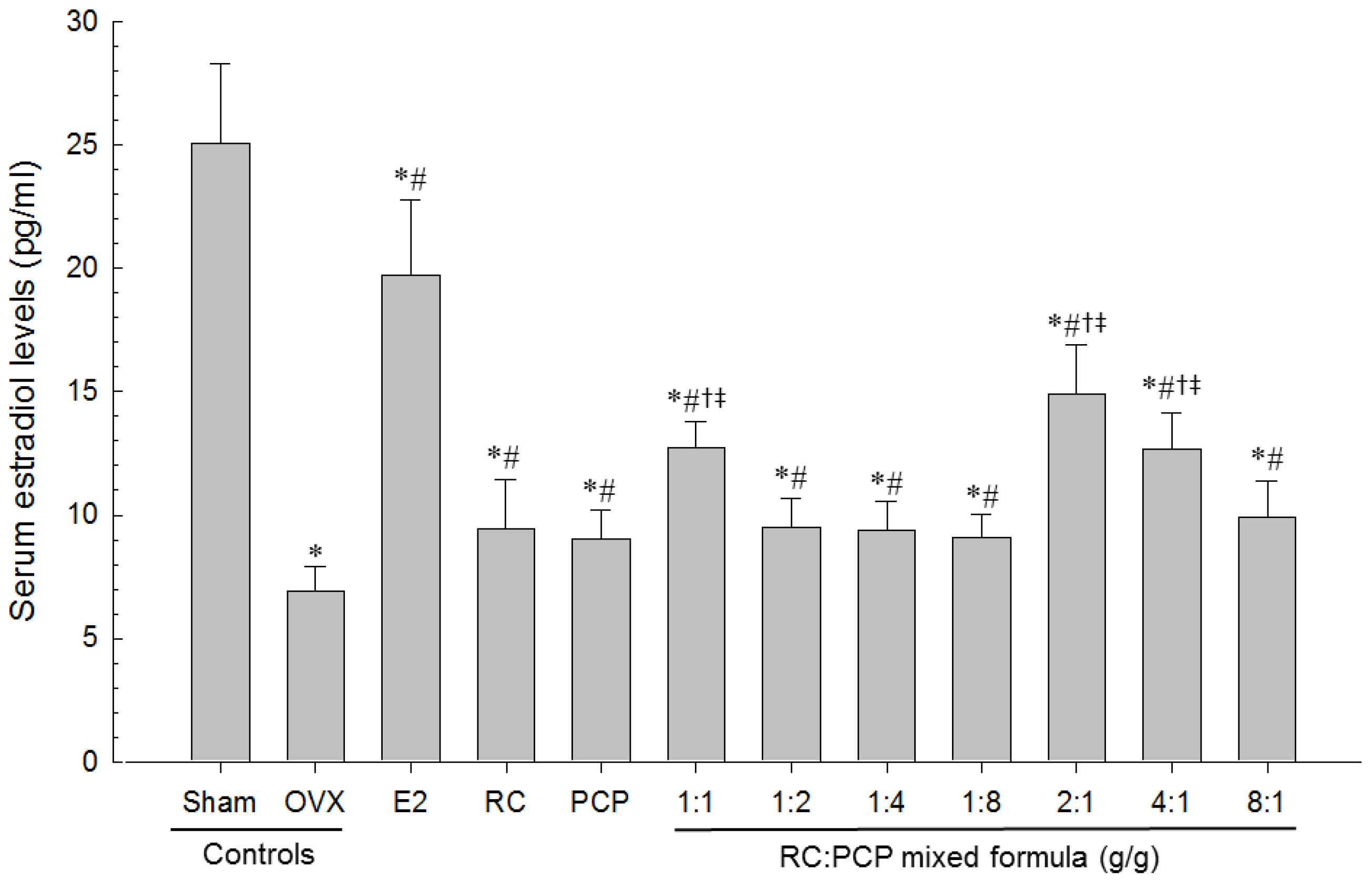
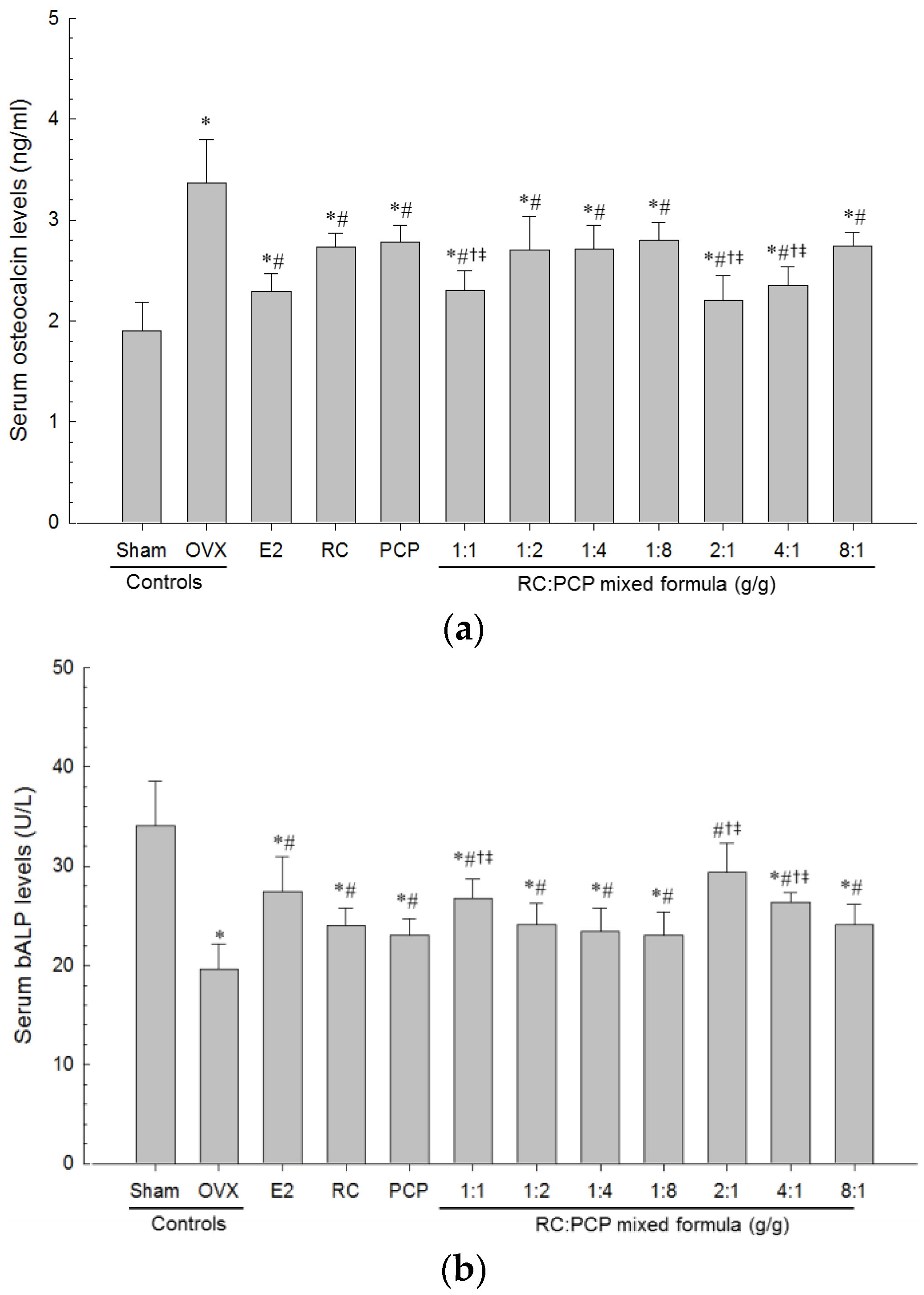
Serum aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), LDL, and triglyceride (TG) levels were detected using an automated blood analyzer (Hemagen Analyst; Hemagen Diagnostic, Columbia, MD, USA), and HDL levels were measured using another typed automated blood analyzer (AU400; Olympus, Tokyo, Japan). In addition, serum osteocalcin levels were detected using a Mouse Osteocalcin ELISA Kit (Immutopics, San Clemente, CA, USA) as ng/mL levels, and serum bALP levels were detected using the Mouse bALP ELISA kit (Quidel Corp., San Diego, CA, USA), as U/L levels as pg/mL, with an ELISA Reader (Tecan, Männedorf, Switzerland). In addition, serum estradiol contents were measured using the chemiluminescent immunoassay technique (ECLIA, Roche e411 immunoassay analyzer, Roche, Penzberg, Germany) from the separated serum at sacrifice in all mice.
2.10. Abdominal Fat Pads, Uterus, and Liver Histological Procedures
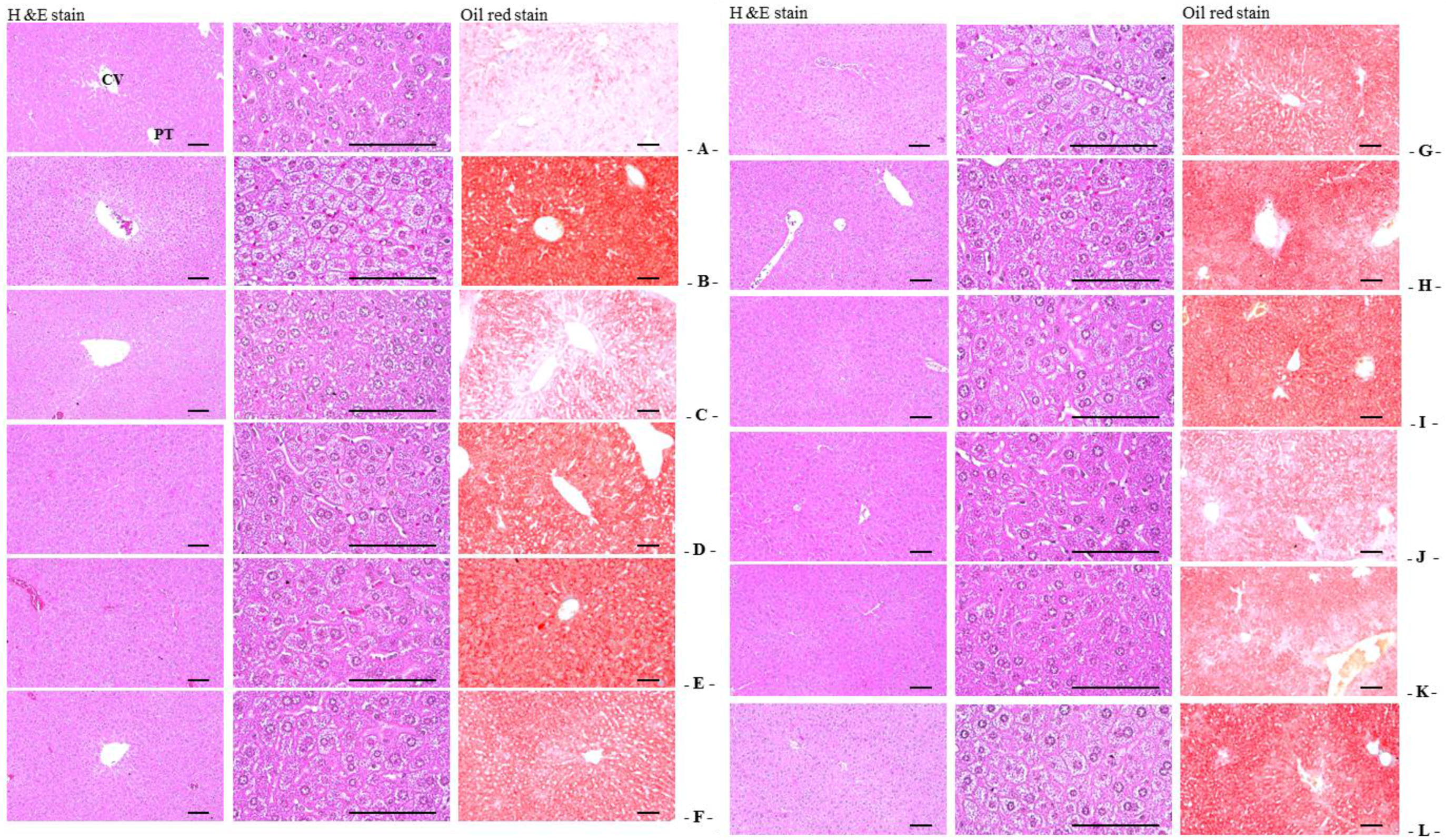
Sampled tissues were fixed in 10% neutral buffered formalin (NBF). After paraffin embedding, 3–4 μm serial sections were prepared. Representative sections were stained with hematoxylin and eosin (H&E) for light microscopic examination. Alternatively, portions of liver that had been dehydrated in 30% sucrose solutions were sectioned by cryostat for staining the lipids with oil red [
33]. The total thicknesses of abdominal fat pads were measured using an automated image analysis processor (
iSolution FL; ver. 9.1, IMT
i-solution Inc., Quebec, QC, Canada) as mm/mouse, and mean diameters of dorsal abdominal white adipocytes were calculated in restricted view fields on a computer monitor, using an automated image analysis processor, as μm. At least 10 white adipocytes per fat pad were considered for histomorphometrical analysis according to our previously established methods [
29,
33,
34]. In addition, total full, mucosa, and epithelial thicknesses of the uterus (μm/uterus) were detected as percentages of uterine glands located in the mucosa (%/mucosa of uterus) using an automated image analyzer. To observe steatosis in the liver, the percentage of fatty change regions in hepatic parenchyma was calculated as percentages between 1 field of liver (%/mm
2 of hepatic parenchyma) under oil red staining, and mean diameters of hepatocytes were calculated in restricted view fields on a computer monitor under H&E staining using an automated image analysis processor, as μm; at least 10 hepatocytes per liver were considered.
2.11. Bone Histological Procedures
The left sides of each mouse femur were separated and fixed in 10% NBF, after which they were decalcified in decalcifying solution (24.4% formic acid and 0.5 N sodium hydroxide) for 3 days (mixed decalcifying solution was exchanged once a day for 3 days). The samples were then embedded in paraffin, sectioned (3~4 μm), and stained with Safranin-O stain. In addition, bone histomorphometry was conducted using an automated image analyzer under microscopy (Nikon, Tokyo, Japan) to examine bone mass and structure with bone resorption in a uniform area of epiphyseal or cortical bone regions of the femur (growth plate regions were excluded). Cortical bone thickness was also measured in the mid-shaft regions of the femur. Trabecular bone volume (TV/BV, TBV; %), thickness of trabecular bone (Tbt; μm/trabecular bone), number (Tbn; mean numbers of trabecular bone/epiphyseal regions), length (Tbl; mm/trabecular bone), and cortical bone thickness (Cbt; μm/mid-shaft cortical bone) were measured for bone mass and structure, and osteoclast cell number (Ocn; mean osteoclast cell numbers/epiphyseal regions) and ratio (OS/BS; %) were measured for bone resorption as described previously [
20,
31,
35].
2.12. Statistical Analyses
All values for the eight mice in this experiment were expressed as means ± SD. Multiple comparison tests were performed a two-tailed test for the different dose groups. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the data were analyzed using the one-way ANOVA test followed by the least-significant differences test to determine which group comparisons were significantly different. When significant deviations from variance homogeneity were observed on the Levene test, the non-parametric the Kruskal-Wallis test was conducted. When a significant difference was observed on the Kruskal-Wallis test, the Mann-Whitney U test was conducted to determine the specific pairs of groups that were significantly different. Statistical analyses were conducted using the SPSS for Windows software package (ver. 14.0; SPSS Inc., Chicago, IL, USA).
4. Discussion
In this study, we found that RC with PCP alleviated climacteric symptoms, such as obesity, hyperlipidemia, hepatic steatosis, and osteoporosis, in OVX mice. Our results indicated that RC:PCP mixed formulae (RC:PCP 2:1, 1:1, and 4:1) exerted enhanced pharmacological effects, compared with RC or PCP alone treatment. Large evidence suggested that pomegranate juice and pomegranate polyphenol extracts can protect women from many types of cancer, cardiovascular disease, diabetes, Alzheimer’s disease, arthritis, and colitis [
25,
36,
37]. In addition, pomegranate seed oil and pomegranate juice including flavonoids and anthocyanidins possessed a strong antioxidant activity when compared to that in red wine or green tea extract [
38,
39]. Flavonoids can interact directly with estrogen receptors and regulate the activity of CYP19, which catalyzes the rate-limiting step in estrogen biosynthesis [
40]. These property results in alterations of the overall hormonal balance, consequently leading to prevent bone loss and reducing osteoporotic effects and other menopausal symptoms [
41]. These evidences support that the favorable effects of RC [
14,
22,
23] and PCP [
26,
39] against OVX-induced symptoms are mainly associated with the antioxidant effects of isoflavonoids [
15,
27]. Therefore, appropriate mixtures consisting of RC and PCP may show greater protective effects by combination of isoflavonoids and ellagic acid [
42,
43]. The RC and PCP mixture may enhance direct free radical scavenging and indirectly induce antioxidative enzymes. It was thus assumed that appropriate RC:PCP mixtures may show more favorable synergistic anti-climacteric effects. Previously, no information to determine the optimal ranges of RC with PCP existed. Our results suggest that among the seven RC:PCP mixed formulae tested, RC:PCP 2:1 mixture showed the greatest inhibition against the climacteric symptoms induced by OVX in this experiment. Thus, our findings are interesting in that PCP exerted a potentially synergistic effect with other antioxidants, such as RC.
OVX induced marked increases in food consumption, body weight and gains, and abdominal fat deposition with adipocyte hypertrophy, which were inhibited significantly by 2:1, 1:1, and 4;1 RC:PCP mixed formulae. The RC:PCP 2:1 mixture showed the strongest inhibitory activities against obesity induced by OVX. Estrogen deficiency induced by OVX markedly accelerated food intake and changes in body fat deposition in the abdominal cavity. Additionally, an accumulation of fat deposition and cellular hypertrophy through expansion of intra-abdominal adipose tissue were observed in obesity-induced OVX mice. The correlation between estradiol and cholecystokinin (CCK) is well documented [
44,
45]. In addition, OVX models have been used to explain the mechanism of the operation of glucagon, because the activities of glucagon and glucagon antibodies on reduced and augmented meal sizes were both enhanced by estradiol [
46]. Estradiol has been regarded as a modulator of eating and body weight by regulating the potency of the feedback signals that control meal size [
46,
47]. Under estradiol deficiency, eating and body weight are increased [
48,
49,
50]. There is evidence of clinical relevance in that postmenopausal women show decreased estradiol levels; also, a high proportion of the obese population is represented by postmenopausal women [
51]. It is assumed that the anti-obesity effects of the RC:PCP mixture may be related to estrogenic food intake effects, but more complex mechanisms are involved in the anti-obesity effects of PCP. Generally, enhanced digestive motility increases in fecal excretion, leading to a reduction in body weight in rodents [
52,
53].
OVX-induced mice showed a significant increase in serum TC, LDL, and TG levels, but decreased serum HDL contents. However, the hyperlipidemia resulting from OVX was inhibited significantly by RC:PCP 2:1, 1:1, and 4:1 mixed formulae. This finding is similar to previous reports showing a significant increase in TC, LDL, and TG, and low HDL levels, in postmenopausal women [
54]; similar trends in serum lipids were observed in OVX induced mice [
55]. The effects of estradiol on serum lipid profiles are believed to be mediated by inhibiting the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) [
56]. Because HMG-CoA is the rate-limiting enzyme involved in cholesterol synthesis, these effects may occur through the elevation of HMG-CoA activity, which is associated with cholesterol synthesis [
56].
OVX-induced liver steatosis was observed, while 2:1, 1:1, and 4:1 RC:PCP mixed formulae inhibited OVX-induced hepatic steatosis significantly. As the liver is the major target organ of HMG-CoA reductase [
34,
57], AST and ALT activities were increased, and hypertrophy and fatty changes were observed in hepatocytes [
58,
59]. These are related to estrogen deficiency-mediated obesity and hyperlipidemia [
34,
59,
60]. Estrogen deficiency is attributed to an atherogenic lipid profile, characterized by HDL-cholesterol, LDL-cholesterol, triglyceride levels [
5], central adiposity [
6], increased diastolic pressure [
7], and increased insulin resistance [
8].
Estrogen-deficiency osteoporosis induced by bilateral OVX was markedly inhibited by RC and PCP single formulae and by the RC:PCP 2:1, 1:1, 4:1 mixed formulae. These effects are related with the increased diversity of isoflavonoids in the mixtures, at least, under these experimental conditions. Previous studies reported that each single formula of RC [
23,
61] or PCP [
26,
39] has anti-osteoporotic effects. Bone loss is aggravated in menopausal women with aging, because of the loss of estrogen. Osteoporosis is a bone disease that occurs due to an imbalance between bone resorption and bone formation, which results in bone loss and structural deterioration of bone [
62]. When determining anti-osteoporotic activities, the increase in bone weight is used as a good marker; however, it is difficult to evaluate anti-osteoporotic agents through changes in bone weight, with the exception of ash bone weight [
63]. For an osteoporosis-related OVX model, bone turnover markers, including serum bALP content and osteocalcin levels, are appropriate [
64,
65,
66], and BMD is considered a pivotal determinant of osteoporosis [
67,
68,
69]. The observation of bone morphology using microscopy is good for evaluating anti-osteoporotic agents [
31,
35,
70,
71], especially, the trabecular and cortical bone changes significant in osteoporotic animals. In addition, some histomorphometric indices of bone mass and bone formation are decreased markedly, whereas histomorphometric indices of bone resorption are increased [
31,
35,
72]. Thus, to evaluate the efficacy of various anti-osteoporosis agents, the histology of bones has been regard as an appropriate approach [
31,
35,
71]. The RC:PCP mixed formulae evaluated here showed anti-osteoporotic activities similar to previous results [
26,
39].
OVX-induced uterine weight along with marked decreases in serum estradiol levels and related uterine atrophic changes, i.e., decreases in total, mucosa and epithelial thicknesses, and uterine glands in the mucosa were suppressed significantly by the RC:PCP mixed formulae. Loss of estrogen after menopause in women leads to climacteric symptoms [
73,
74], because estrogens affect numerous female target organs, such as the uterus, vagina, and skeletal and cardiovascular systems [
75,
76]. Recent study reported that soy isoflavone supplementation has no influence in endometrial thickness or in the rates of endometrial hyperplasia and female cancer in postmenopausal women [
24]. In contrast, the other study reported that genistein (0.7 mg per day) had protective effect on trabecular bone loss in OVX control mice without hypertrophic effects on the uterus, while administration of genistein (5 mg per day) resulted in uterine hypertrophy. These data indicate that there is a marked difference between genistein dosages that prevent bone loss and those that induce uterine hypertrophy [
77]. Genestein and specific ERβ agonist consumption may prevent the ileal and colonic epithelium from tumor development via modulation of tissue homeostasis [
78]. Genistein enhanced the basal Toll like receptor 2 (TLR2) and reduced the viral component-induced TLR2 protein expression in human endometrial epithelial cells may suggest the potential role of this soy isoflavone in stimulating the uterine immune function and probably attenuating the inflammation of endometrium following pathogen [
79]. Isoflavonoids increase in uterine masses via uterine water imbibition and/or cell proliferation [
75,
80], which are mediated by ERα [
81,
82,
83]. However, there had limitation in this study that the increase of estradiol level was not explained clearly, more detail mechanism should be studied in future.