Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Subjects
2.2. Study Procedures
2.3. Statistical Analyses
2.4. Sample-Size Calculation
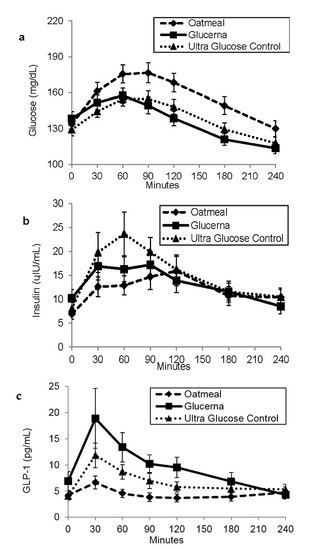
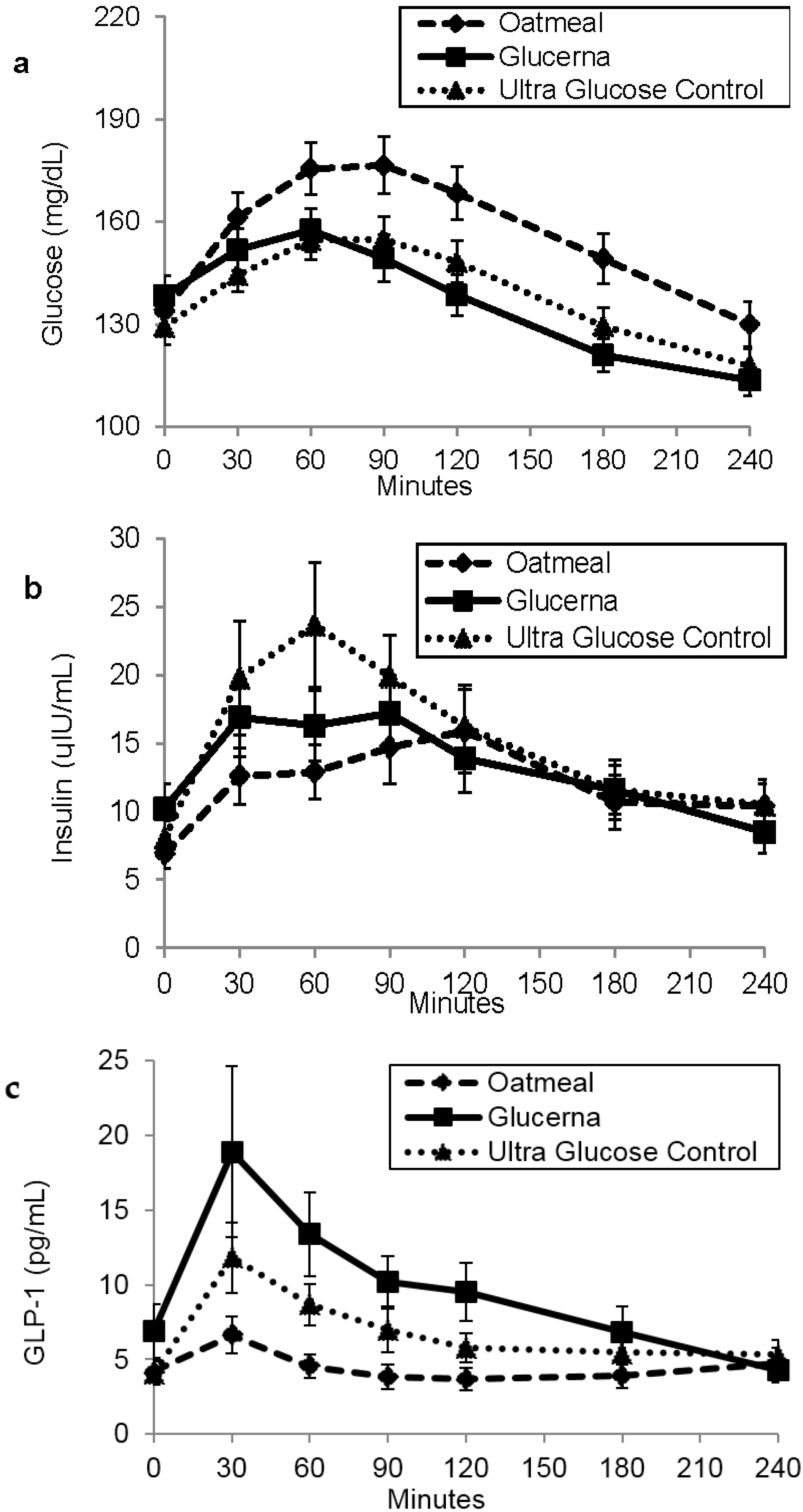
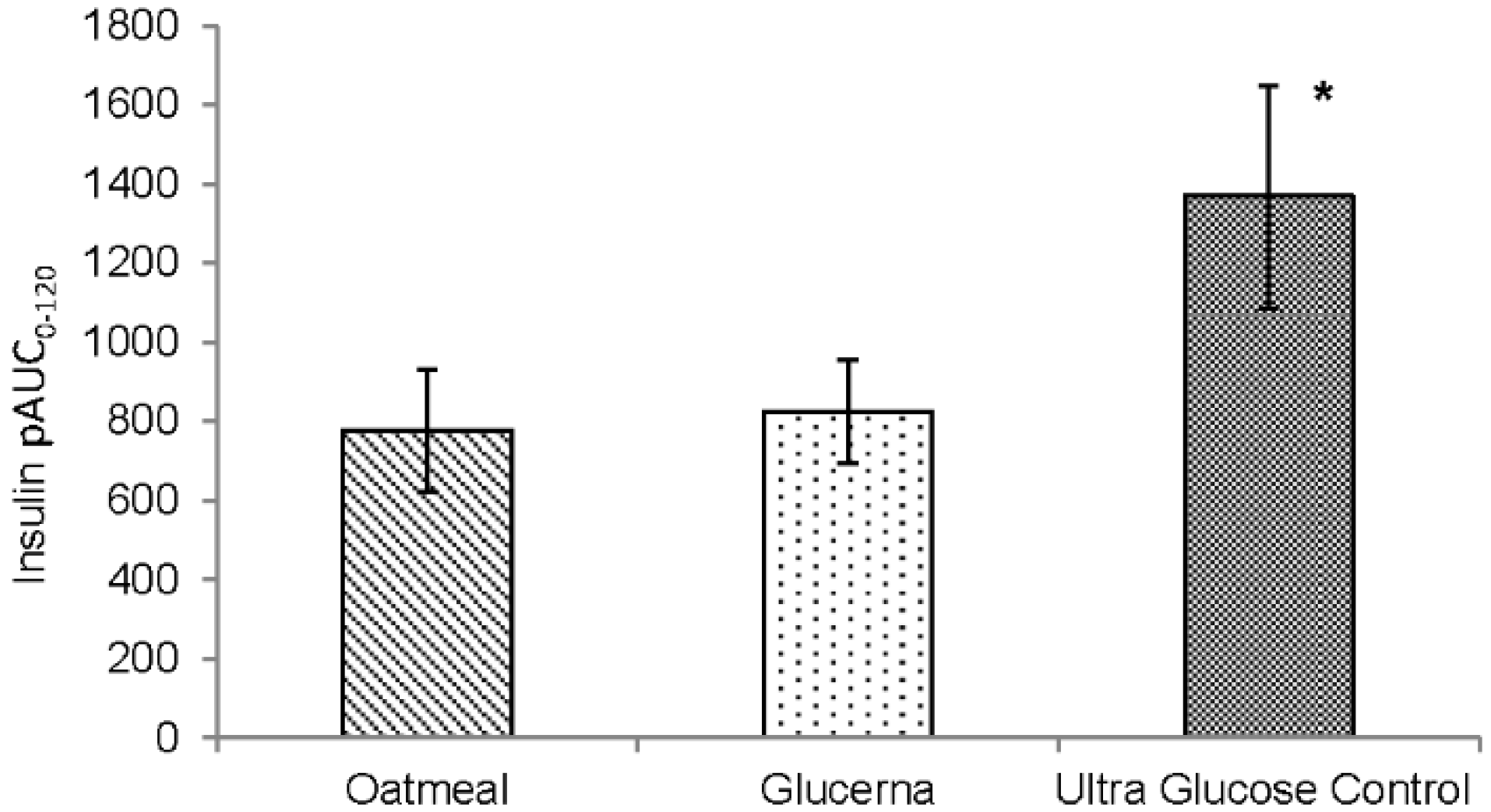
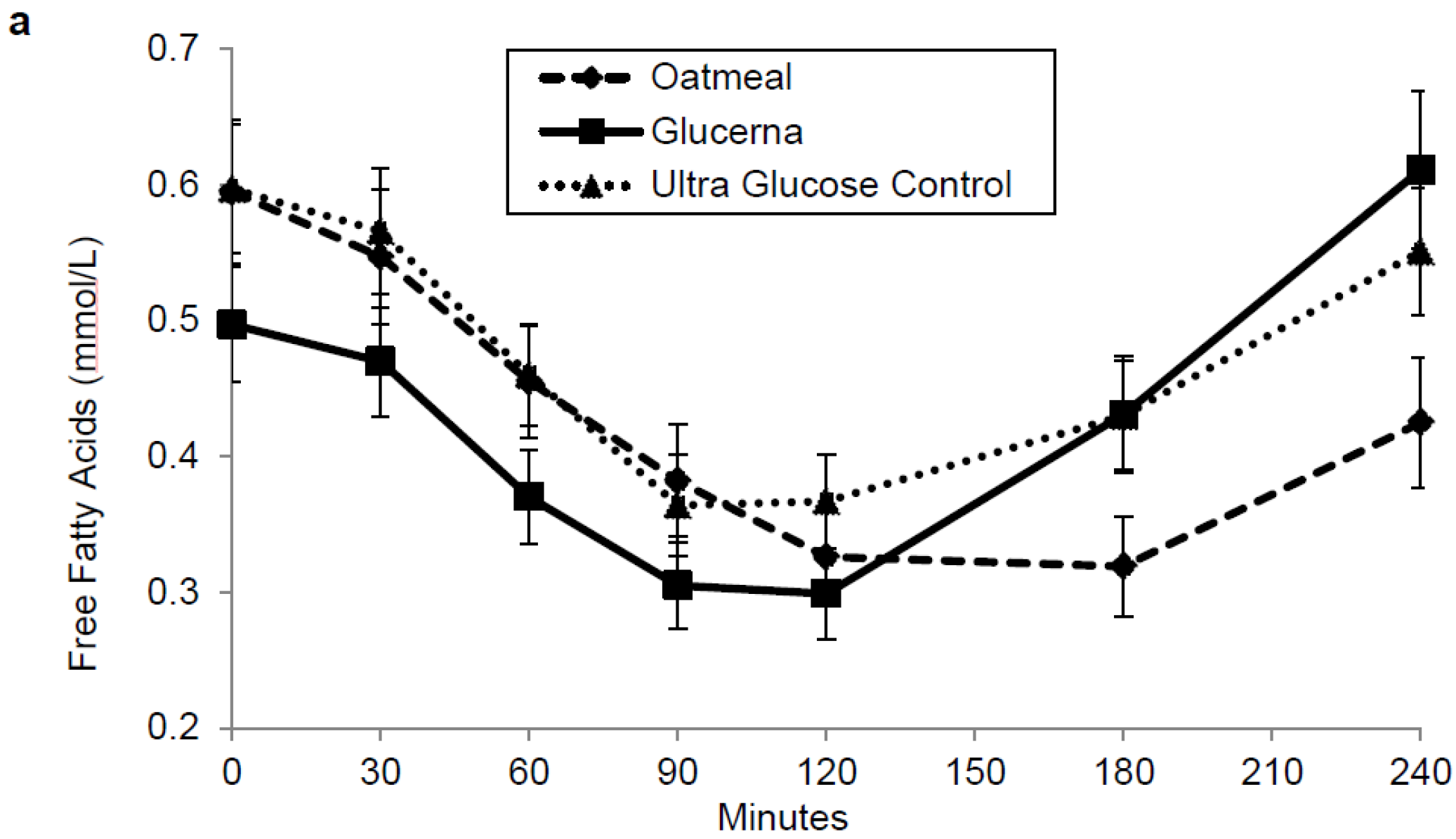
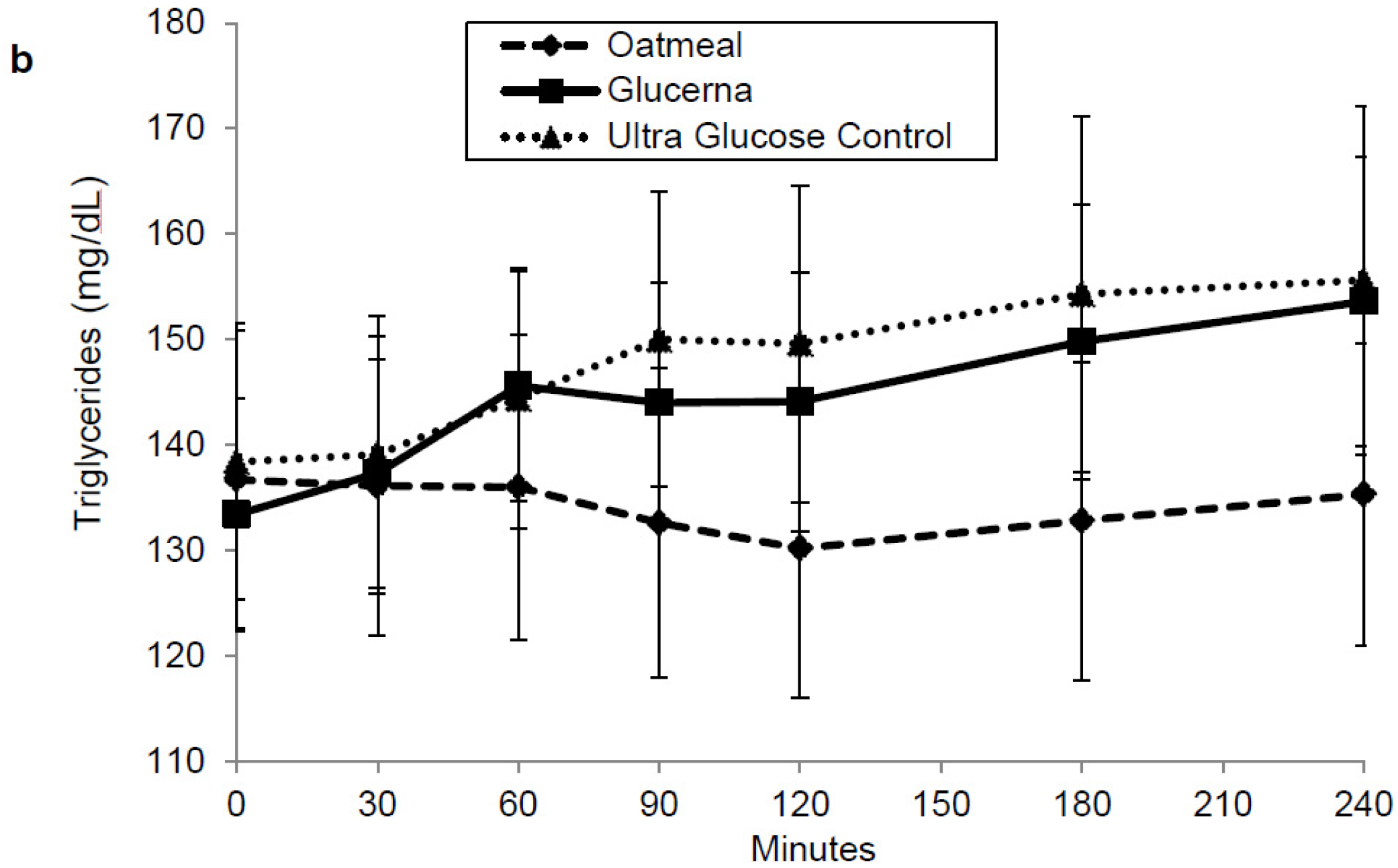
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| DSNFs | diabetes-specific nutritional formulas |
| FFA | free fatty acids |
| GLP-1 | glucagon-like peptide-1 |
| GL | Glucerna |
| MNT | medical nutrition therapy |
| MUFA | monounsaturated fatty acids |
| OM | oatmeal |
| PP | postprandial |
| SEM | standard error of mean |
| TG | triglycerides |
| UGC | Ultra Glucose Control |
References
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. American Diabetes A, European Association for the Study of D: Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009, 52, 17–30. [Google Scholar] [PubMed]
- Morris, S.F.; Wylie-Rosett, J. Medical nutrition therapy: A key to diabetes management and prevention. Clin. Diabetes 2010, 28, 12. [Google Scholar] [CrossRef]
- Elia, M.; Ceriello, A.; Laube, H.; Sinclair, A.J.; Engfer, M.; Stratton, R.J. Enteral Nutritional Support and Use of Diabetes-Specific Formulas for Patients with Diabetes: A systematic review and meta-analysis. Diabetes Care 2005, 28, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; West, D.S.; Neiberg, R.H.; Wing, R.R.; Ryan, D.H.; Johnson, K.C.; Foreyt, J.P.; Hill, J.O.; Trence, D.L.; Vitolins, M.Z.; et al. One-year weight losses in the Look AHEAD study: Factors associated with success. Obesity 2009, 17, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Neiberg, R.H.; Wing, R.R.; Clark, J.M.; Delahanty, L.M.; Hill, J.O.; Krakoff, J.; Otto, A.; Ryan, D.H.; Vitolins, M.Z.; et al. Four-year weight losses in the Look AHEAD study: Factors associated with long-term success. Obesity 2011, 19, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515. [Google Scholar] [CrossRef] [PubMed]
- Dailey, M.J.; Moran, T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol. Metab. 2013, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Krarup, T.; Deacon, C.F.; Madsbad, S.; Holst, J.J. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001, 50, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Holst, J.J.; Willms, B.; Schmiegel, W. Glucagon-like peptide 1 (GLP-1) as a new therapeutic approach for type 2-diabetes. Exp. Clin. Endocrinol. Diabetes 1996, 105, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B. Glucagon-like peptide-1 (GLP-1): A gut hormone of potential interest in the treatment of diabetes. Bioessays 1998, 20, 642–651. [Google Scholar] [CrossRef]
- Ahrén, B.; Schmitz, O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm. Metab. Res. 2003, 36, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Devitt, A.; Jeffery Oliver, S.; Refaat Hegazi, A.; Mustad, V. Glycemia Targeted Specialized Nutrition (GTSN) improves postprandial glycemia and GLP-1 with similar appetitive responses compared to a healthful whole food breakfast in persons with type 2 diabetes: A randomized, controlled trial. J. Diabetes Res. Clin. Metab. 2012, 1, 20. [Google Scholar] [CrossRef]
- Voss, A.C.; Maki, K.C.; Garvey, W.T.; Hustead, D.S.; Alish, C.; Fix, B.; Mustad, V.A. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008, 24, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Sadiq Butt, M.; Tahir-Nadeem, M.; Khan, M.K.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Tapola, N.; Karvonen, H.; Niskanen, L.; Mikola, M.; Sarkkinen, E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Gügolz, E.; Würsch, P. Effects of breakfast cereals containing various amounts of β-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care 1996, 19, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Hernandez-Salazar, E.; Kam-Ramos, A.M.; Robles-Cervantes, J.A. Effect of a nutritional liquid supplement designed for the patient with diabetes mellitus (Glucerna SR) on the postprandial glucose state, insulin secretion and insulin sensitivity in healthy subjects. Diabetes Obes. Metab. 2006, 8, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Devitt, A.A.; Williams, J.A.; Choe, Y.S.; Hustead, D.S.; Mustad, V.A. Glycemic responses to glycemia-targeted specialized-nutrition beverages with varying carbohydrates compared to a standard nutritional beverage in adults with type 2 diabetes. Adv. Biosci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Heine, R.; Dekker, J. Beyond postprandial hyperglycaemia: Metabolic factors associated with cardiovascular disease. Diabetologia 2002, 45, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Nalysnyk, L.; Hernandez-Medina, M.; Krishnarajah, G. Glycaemic variability and complications in patients with diabetes mellitus: Evidence from a systematic review of the literature. Diabetes Obes. Metab. 2010, 12, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial Dysmetabolism and Oxidative Stress in Type 2 Diabetes: Pathogenetic Mechanisms and Therapeutic Strategies. Med. Res. Rev. 2015, 35, 968–1031. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. International Prandial Glucose Regulation Study G: Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.; Krezowski, P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Kruijshoop, M.; Menheere, P.P.; Wagenmakers, A.J.; Saris, W.H.; Keizer, H.A. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003, 26, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Underhill, L.H.; Polonsky, K.S.; Sturis, J.; Bell, G.I. Non-insulin-dependent diabetes mellitus—A genetically programmed failure of the beta cell to compensate for insulin resistance. N. Engl. J. Med. 1996, 334, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Manders, R.J.; Wagenmakers, A.J.; Koopman, R.; Zorenc, A.H.; Menheere, P.P.; Schaper, N.C.; Saris, W.H.; van Loon, L.J. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am. J. Clin. Nutr. 2005, 82, 76–83. [Google Scholar] [PubMed]
- Wolever, T.M.; Bentum-Williams, A.; Jenkins, D.J. Physiological modulation of plasma free fatty acid concentrations by diet: Metabolic implications in nondiabetic subjects. Diabetes Care 1995, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Storm, H.; Holst, J.J.; Hermansen, K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [PubMed]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated Fatty Acid Diets Improve Glycemic Tolerance through Increased Secretion of Glucagon-Like Peptide-1. Endocrinology 2001, 142, 1148–1155. [Google Scholar] [PubMed]
| Gender | |
|---|---|
| Male | 12 (54.6%) |
| Female | 10 (45.5%) |
| Age (years) | 62.3 ± 6.8 |
| Height (cm) | 171.1 ± 8.9 |
| Weight (kg) | 97.4 ± 21.3 |
| BMI (kg/m2) | 33.2 ± 5.9 |
| Diabetes duration (years) | 9.5 ± 9.8 |
| HbA1c (%) | 6.8 ± 0.7 |
| Participants using diabetes medications (n) | 19 |
| Participants using DPP-4 inhibitors | 6 |
| Participants using lipid lowering medications | 15 |
| Oatmeal (OM) | Glucerna (GL) | Ultra Glucose Control (UGC) | ||||
|---|---|---|---|---|---|---|
| Amount | % DV | Amount | % DV | Amount | % DV | |
| Serving Size | 53.3 (g) | NA | 8 (fl oz) | NA | 56 (g) | NA |
| Energy (kcal) | 200 | 10 | 200 | 10 | 200 | 10 |
| Total Fat (g) | 4 | 6.7 | 7 | 11 | 7 | 11 |
| % Energy | 18 | - | 32 | - | 32 | - |
| Saturated Fat (g) | 0 | 0 | 0.5 | 3 | 1 | 5 |
| Monounsaturated Fat (g) | 1.3 | - | 5.2 | - | 4.5 | - |
| Total Carbohydrates (g) | 36 | 12 | 26 | 9 | 27 | 9 |
| % Energy | 72 | - | 52 | - | 54 | - |
| Dietary Fiber (g) | 5.3 | 20 | 3 | 12 | 3 | 12 |
| Protein (g) | 6.7 | NA | 10 | 20 | 15 | 30 |
| % Energy | 13 | - | 20 | - | 30 | - |
| Amino Acid | Oatmeal a | Glucerna b | Ultra Glucose Control b |
|---|---|---|---|
| Histidine | 0.164 | 0.327 | 0.329 |
| Isoleucine | 0.295 | 0.603 | 1.119 |
| Leucine | 0.567 | 1.169 | 1.850 |
| Lysine | 0.290 | 0.946 | 0.872 |
| Methionine | 0.131 | 0.312 | 0.175 |
| Phenylalanine | 0.391 | 0.610 | 0.731 |
| Threonine | 0.259 | 0.655 | 0.518 |
| Tryptophan | - | 0.154 | 0.145 |
| Valine | 0.398 | 0.733 | 1.183 |
| Alanine | 0.354 | 0.442 | 0.613 |
| Arginine | 0.491 | 0.473 | 1.167 |
| Aspartic acid | 0.602 | 1.027 | 1.492 |
| Cystine | 0.208 | 0.076 | 0.160 |
| Glutamic acid | 1.635 | 2.650 | 2.261 |
| Glycine | 0.367 | 0.274 | 0.560 |
| Proline | 0.405 | 1.126 | 0.604 |
| Serine | 0.367 | 0.710 | 0.698 |
| Tyrosine | 0.257 | 0.544 | 0.540 |
| Oatmeal (OM) | Glucerna (GL) | Ultra Glucose Control (UGC) | |
|---|---|---|---|
| Glucose (mg·min/dL) | 37,828.6 ± 1678.7 | 32,747.0 ± 1287.7 * | 33,538.6 ± 1266.5 * |
| Insulin (IU·min/mL) | 2995.1 ± 442.4 | 3247.3 ± 538.9 | 3799.6 ± 620.9 |
| Active GLP-1 (pg·min/mL) | 1055.5 ± 187.3 | 2347.7 ± 464.2 * | 1631.5 ± 246.8 * |
| Free fatty acids (mmol·min/L) | 97.023 ± 8.7 | 99.2 ± 8.5 | 108.4 ± 9.0 |
| Triglyceride (mg·min/dL) | 32,077.5 ± 3517.6 | 34,887.3 ± 2857.0 | 35,739.5 ± 3438.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottalib, A.; Mohd-Yusof, B.-N.; Shehabeldin, M.; Pober, D.M.; Mitri, J.; Hamdy, O. Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients 2016, 8, 443. https://doi.org/10.3390/nu8070443
Mottalib A, Mohd-Yusof B-N, Shehabeldin M, Pober DM, Mitri J, Hamdy O. Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients. 2016; 8(7):443. https://doi.org/10.3390/nu8070443
Chicago/Turabian StyleMottalib, Adham, Barakatun-Nisak Mohd-Yusof, Mohamed Shehabeldin, David M. Pober, Joanna Mitri, and Osama Hamdy. 2016. "Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia" Nutrients 8, no. 7: 443. https://doi.org/10.3390/nu8070443