Osteoporosis: Modern Paradigms for Last Century’s Bones †
Abstract
:1. Introduction
2. Review
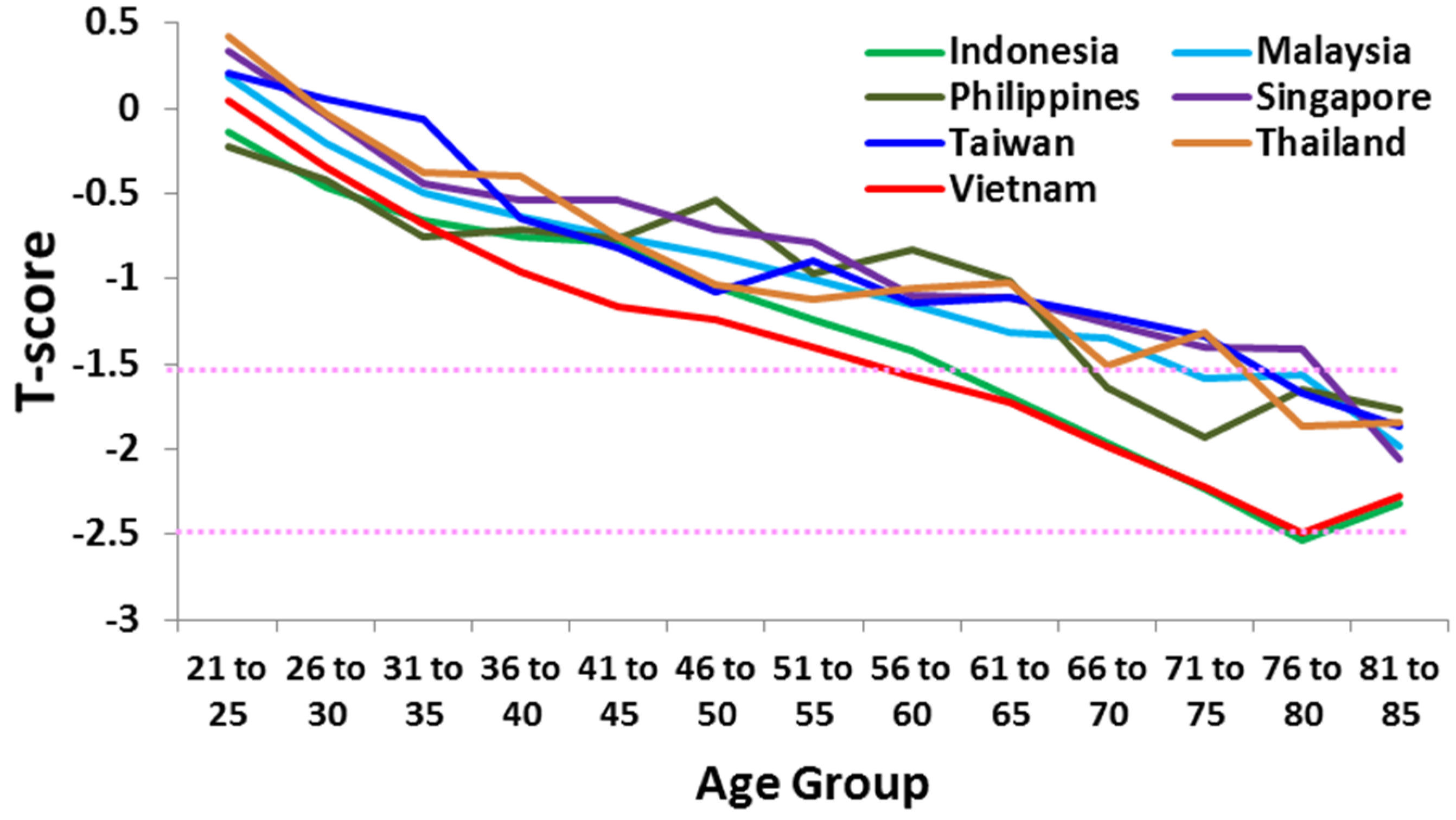
2.1. Ethnicity
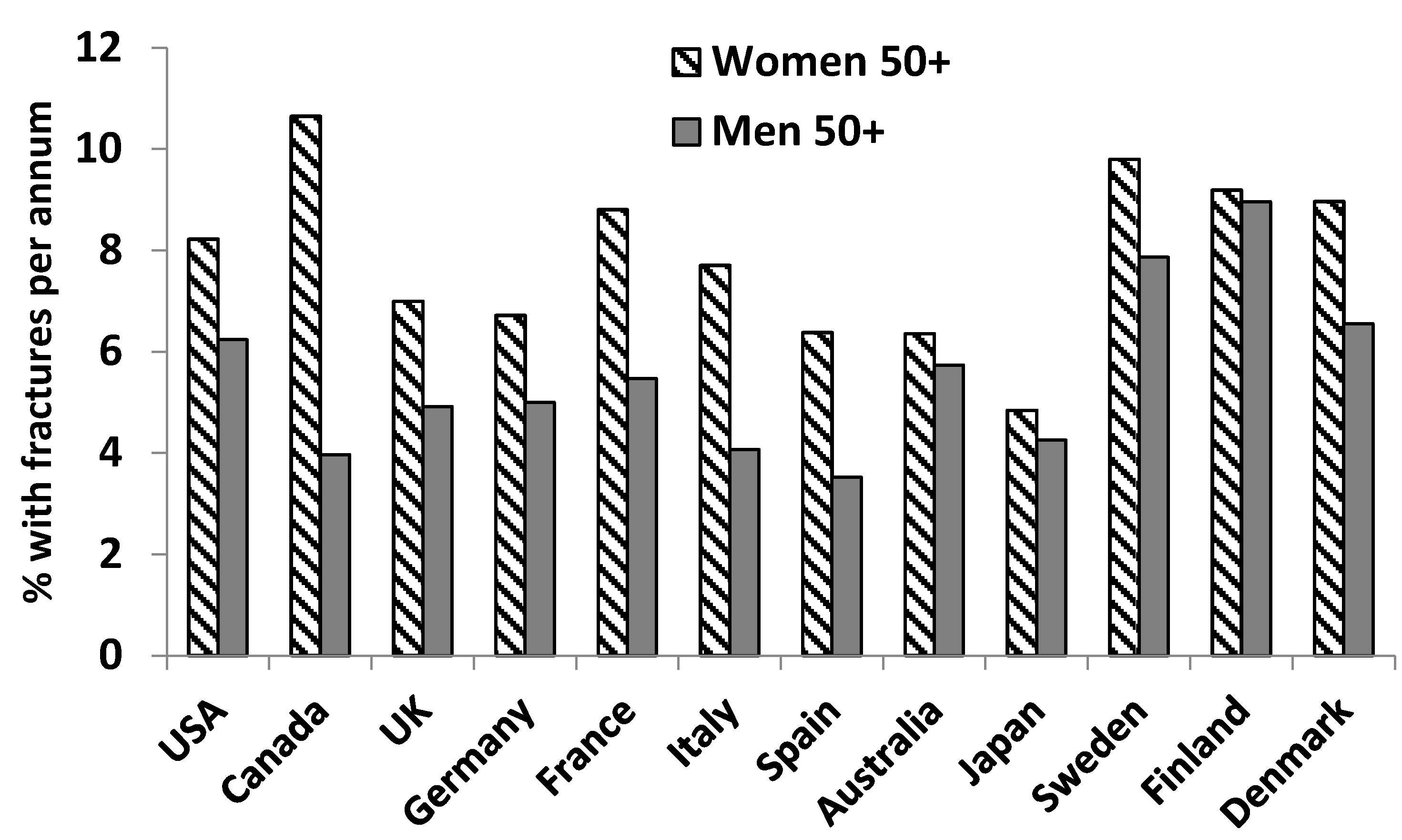
2.2. Sex
2.3. Age
2.4. Body Phenotype
2.5. Oestrogen
2.6. Calcium
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jilka, R.L.; O’Brien, C.A. The role of osteocytes in age-related bone loss. Curr. Osteoporos. Rep. 2016, 14, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Alarkawi, D.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Accelerated bone loss and increased post-fracture mortality in elderly men and women. Osteoporos. Int. 2015, 6, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Kijowski, R.; Tuite, M.; Kruger, D.; Munoz Del Rio, A.; Kleerekoper, M.; Binkley, N. Evaluation of trabecular microarchitecture in nonosteoporotic postmenopausal women with and without fracture. J. Bone Miner. Res. 2012, 27, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Fraser, J. Diagnosis and management of osteoporosis. Practitioner 2015, 259, 15–19. [Google Scholar] [PubMed]
- Popp, A.W.; Meer, S.; Krieg, M.A.; Perrelet, R.; Hans, D.; Lippuner, K. Bone mineral density (BMD) and vertebral trabecular bone score (TBS) for the identification of elderly women at high risk for fracture: The SEMOF cohort study. Eur. Spine J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.W.; Strader, C.; Fitzpatrick, L.A.; Anthony, M.S. Sex- and age-specific incidence of non-traumatic fractures in selected industrialized countries. Arch. Osteoporos. 2012, 7, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; van de Langerijt, L.; Watts, N.B.; Eastell, R.; Genant, H.; Grauer, A.; Cahall, D.L.; IMPACT Study Group. Underdiagnosis of vertebral fractures is a worldwide problem: The IMPACT study. J. Bone Miner. Res. 2005, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; McNeill, R.; Leung, W.; Tadwan, E.; Willingale, J. Current and future economic burden of osteoporosis in New Zealand. Appl. Health Econ. Health Policy 2011, 9, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Todd, J.M.; Schollum, L.M.; Kuhn-Sherlock, B.; McLean, D.W.; Wylie, K. Bone health comparison in seven Asian countries using calcaneal ultrasound. BMC Musculoskelet. Disord. 2013, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.V.; Mandlik, R.M. Epidemiology and treatment of osteoporosis in women: An Indian perspective. Int. J. Women’s Health 2015, 7, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Tsukutani, Y.; Hagino, H.; Ito, Y.; Nagashima, H. Epidemiology of fragility fractures in Sakaiminato, Japan: Incidence, secular trends, and prognosis. Osteoporos. Int. 2015, 26, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Lofthus, C.M.; Frihagen, F.; Meyer, H.E.; Nordsletten, L.; Melhuus, K.; Falch, J.A. Epidemiology of distal forearm fractures in Oslo, Norway. Osteoporos. Int. 2008, 19, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Odén, A.; Lorentzon, M.; McCloskey, E.; Kanis, J.A.; Harvey, N.C.; Karlsson, M.K.; Mellström, D. Is the Swedish FRAX model appropriate for Swedish immigrants? Osteoporos. Int. 2015, 26, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Cole, Z.A.; Holroyd, C.R.; Earl, S.C.; Harvey, N.C.; Dennison, E.M.; Melton, L.J.; Cummings, S.R.; Kanis, J.A.; IOF CSA Working Group on Fracture Epidemiology. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 2011, 22, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Massey University, Palmerston North, New Zealand. Unpublished data. 2013.
- Kanis, J.A.; Oden, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef] [PubMed]
- Omsland, T.K.; Emaus, N.; Tell, G.S.; Magnus, J.H.; Ahmed, L.A.; Holvik, K.; Center, J.; Forsmo, S.; Gjesdal, C.G.; Schei, B.; et al. Mortality following the first hip fracture in Norwegian women and men (1999–2008). A NOREPOS study. Bone 2014, 63, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Brozek, W.; Reichardt, B.; Kimberger, O.; Zwerina, J.; Dimai, H.P.; Klaushofer, K.; Zwettler, E. Mortality after hip fracture in Austria 2008–2011. Calcif. Tissue Int. 2014, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Sandström, L.; McGuigan, F.E.; Callréus, M.; Akesson, K.E. Peak Bone Mass and Quantitative Ultrasound Bone Properties in Young Adulthood: A Study in the PEAK-25 Cohort of Women. J. Clin. Densitom. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P.; Chevalley, T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr. Rev. 2014, 35, 820–847. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Bonjour, J.P.; Ferrari, S.; Rizzoli, R. Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporos. Int. 2011, 22, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Buttazzoni, C.; Rosengren, B.E.; Karlsson, C.; Dencker, M.; Nilsson, J.A.; Karlsson, M.K. A pediatric bone mass scan has poor ability to predict peak bone mass: An 11 year prospective study in 121 children. Calcif. Tissue Int. 2015, 96, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Parallels between nutrition and physical activity: Research questions in development of peak bone mass. Res. Q. Exerc. Sport 2015, 86, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Julian-Almarcegui, C.; Gomez-Cabello, A.; Huybrechts, I.; Gonzalez-Aguero, A.; Kaufman, J.M.; Casajus, J.A.; Vincente-Rodriguez, G. Combined effects of interaction between physical activity and nutrition on bone health in children and adolescents: A systematic review. Nutr. Rev. 2015, 73, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.; Thiex, N.W.; Sudhagoni, R.G. Does exercise influence pediatric bone? A systematic review. Clin. Orthop. Relat. Res. 2015, 473, 3658–3672. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Aldridge, V.; Clark, E.M.; Misra, M.; Micali, N. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos. Int. 2016, 27, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Ribot, C.; Tremollieres, F.; Pouilles, J.M. The effect of obesity on postmenopausal bone loss and the risk of osteoporosis. Adv. Nutr. Res. 1994, 9, 257–271. [Google Scholar] [PubMed]
- Jensen, G.F. Osteoporosis of the slender smoker revisited by then epidemiologic approach. Eur. J. Clin. Investig. 1986, 16, 239–242. [Google Scholar] [CrossRef]
- Korpelainen, R.; Korpelainen, J.; Heikkinen, J.; Vaananen, K.; Keinanen-Kiukaanniemi, S. Lifestyle factors are associated with osteoporosis in lean women but not in normal and overweight women: A population-based cohort study of 1222 women. Osteoporos. Int. 2003, 14, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kujath, A.S.; Quinn, L.; Elliot, M.E.; LeCaire, T.J.; Binkley, N.; Molino, A.R.; Danielson, K.K. Different health behaviours and clinical factors associated with bone mineral density and bone turnover in premenopausal women with and without type 1 diabetes. Diabetes Metab. Res. Rev. 2015, 4, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Johnson, M.A.; Lewis, R.D.; Heymsfield, S.B.; Chowdhury, H.A.; Modlesky, C.M.; Shapses, S.A. Bone turnover and body weight relationships differ in normal weight compared to heavier postmenopausal women. Osteoporos. Int. 2003, 14, 116–122. [Google Scholar] [PubMed]
- Glass, N.A.; Torner, J.C.; Letuchy, E.M.; Burns, T.L.; Janz, K.F.; Eichenberger, G.J.M.; Schlechte, J.A.; Levy, S.M. The relationship between greater pre-pubertal adiposity, subsequent age of maturation and bone strength during adolescence. J. Bone Miner. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sotunde, O.F.; Kruger, H.S.; Wright, H.H.; Havemann-Nel, L.; Kruger, I.M.; Wentzel-Viljoen, E.; Kruger, A.; Tieland, M. Lean mass appears to be more strongly associated with bone health than fat mass in urban black South African women. J. Nutr. Health Aging 2015, 19, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Pavel, O.R.; Popescu, M.; Novac, L.; Mogoantă, L.; Pavel, L.P.; Vicaş, R.M.; Trăistaru, M.R. Postmenopausal osteoporosis—Clinical, biological and histopathological aspects. Romanian J. Morphol. Embryol. 2016, 57, 121–130. [Google Scholar]
- Goh, V.H.; Hart, W.G. Aging and bone health in Singaporean Chinese pre-menopausal and postmenopausal women. Maturitas 2016, 89, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, L.E.; Nachtigall, R.H.; Nachtigall, R.D.; Beckman, E.M. Estrogen replacement therapy I: A 10-year prospective study in the relationship to osteoporosis. Obstet. Gynecol. 1979, 53, 277–281. [Google Scholar] [PubMed]
- Stevenson, J.C. Prevention of osteoporosis: One step forward, two steps back. Menopause Int. 2011, 17, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Galid, A.; Kubista, E. Estrogen replacement therapy in women with a history of breast cancer. Maturitas 1999, 32, 63–68. [Google Scholar] [CrossRef]
- Lupo, M.; Dains, J.E.; Madsen, L.T. Hormone Replacement Therapy: An Increased Risk of Recurrence and Mortality for Breast Cancer Patients? J. Adv. Pract. Oncol. 2015, 6, 322–330. [Google Scholar] [PubMed]
- Shim, S.H.; Lee, S.J.; Kim, S.N. Effects of hormone replacement therapy on the rate of recurrence in endometrial cancer survivors: A meta-analysis. Eur. J. Cancer 2014, 50, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ding, C.Y.; Qiu, L.H. Postoperative hormone replacement therapy for epithelial ovarian cancer patients: A systematic review and meta-analysis. Gynecol. Oncol. 2015, 139, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kim, E.H. Hormonal Replacement Therapy and the Risk of Lung Cancer in Women: An Adaptive Meta-analysis of Cohort Studies. J. Prev. Med. Public Health 2015, 48, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.; Lam, S.; Chen, Y.; Sham, A.; Lau, J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos. Int. 2003, 14, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Onouchi, T.; Takahashi, M.; Ito, H.; Orimo, H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos. Int. 2000, 11, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Dalais, F.S.; Ebeling, P.R.; Kotsopoulos, D.; McGrath, B.P.; Teede, H.J. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin. Endocrinol. 2003, 58, 704–709. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hisada, A.; Yoshinaga, J. Inter- and intra-individual variation in urinary excretion of daidzein and equol in female Japanese. Biomarkers 2014, 19, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F. The role of colonic bacteria in the metabolism of natural isoflavone daidzin to equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Utian, W.H.; Jones, M.; Setchell, K.D. S-equol: A potential nonhormonal agent for menopause-related symptom relief. J. Women’s Health (Larchmt.) 2015, 24, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Van der Velpen, V.; Geelen, A.; Hollman, P.C.; Schouten, E.G.; Van’t Veer, P.; Afman, L.A. Isoflavone supplement composition and equol producer status affect gene expression in adipose tissue: A double-blind, randomized, placebo-controlled crossover trial in postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Peiroten, A.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of latic acid bacteria and bifidobacteria with biotechnical interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Hori, S.; Nakagawa, H.; Katada, K.; Kamada, K.; Uchiyama, K.; Handa, O.; Takagi, T.; Naito, Y.; Yoshikawa, T. Relationships among fecal daidzein metabolites, dietary habit and BMI in healthy volunteers: A preliminary study. Biosci. Microbiota Food Health 2015, 34, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Galandi, S.L.; Summer, S.S.; Zhao, X.; Heubi, J.E.; King, E.C.; Setchell, K.D. S-(−) equol production is developmentally regulated and related to early diet composition. Nutr. Res. 2014, 34, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Nakaktsu, C.H.; Armstrong, A.; Clavijo, A.P.; Martin, B.R.; Barnes, S.; Weaver, C.M. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS ONE 2014, 9, e108924. [Google Scholar]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Choi, H.S.; Choi, M.J.; Chung, H.Y. Calcium and vitamin D supplementations: 2015 position statement of the Korean Society for Bone and Mineral Research. J. Bone Metab. 2015, 22, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Fan, S.G.; Li, S.L.; Chen, Y.S.; Wu, H.; Guo, Y.L. Low 25(OH)D serum levels are related with hip fracture in postmenopausal women: A matched case-control study. J. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D signalling in calcium and bone homeostasis: A delicate balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Godala, M.; Materek-Kusmierkiewicz, I.; Moczulski, D.; Gaszynska, E.; Szatko, F.; Tokarski, S.; Kowalski, J. Assessment of 25(OH)D vitamin concentration in plasma of residents of Lodz with metabolic syndrome in pre- and postmenopausal period. Prz. Meopauzalny 2014, 13, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G. Vitamin D status among healthy postmenopausal women in South Ameica. Dermato-Endocrin 2013, 5, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, A.; Horvath, A.; Szczechura, M.; Kaminska, M.; Dziechciarz, P. High prevalence of vitamin D insufficiency in community-dwelling postmenopausal Polish women. Prz. Menopauzalny 2014, 13, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; Bruce-Mensah, A.; Whitmire, M.; Rizvi, A.A. Hypercalcemia associated with calcium supplement use: Prevalence and characteristics in hospitalised patients. J. Clin. Med. 2015, 4, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S. Calcium supplements: Do they help or harm? Menopause 2014, 21, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, R.; Iozzino, M.; D’onofrio, L.; Terrinoni, I.; Maddaloni, E.; Casini, A.; Campagna, G.; Santonati, A.; Palermo, A. Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis. Minerva Endocrinol. 2015, 40, 231–237. [Google Scholar] [PubMed]
- Reid, I.R. Should we prescribe calcium supplements for osteoporosis prevention? J. Bone Metab. 2014, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Abrams, S.A.; Committee on Nutrition. Optimising bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bistow, S.M.; Bolland, M.J. Calcium supplements: Benefits and risks. J. Intern. Med. 2015, 278, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.; Jarjou, L.M.; Zhou, B.; Prentice, A.; Schoenmakers, I. Ethnic differences in calcium, phosphate and bone metabolism. Proc. Nutr. Soc. 2014, 73, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Novotny, R.; Bilezikian, J.P.; Weaver, C.M. Race and diet interactions in the acquisition, maintenance, and loss of bone. J. Nutr. 2008, 138, 1256S–1260S. [Google Scholar] [PubMed]
- Freedman, B.I.; Register, T.C. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat. Rev. Nephrol. 2012, 12, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Martin, B.R.; Braun, M.M.; Wastney, M.E.; McCabe, G.P.; McCabe, L.D.; DiMeglio, L.A.; Peacock, M.; Weaver, C.M. Calcium requirements and metabolism in Chinese-American boys and girls. J. Bone Miner. Res. 2010, 25, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; von Hurst, P.R.; Booth, C.L.; Kuhn-Sherlock, B.; Todd, J.M.; Schollum, L.M. Postprandial metabolic responses of serum calcium, parathyroid hormone and C-telopeptide of type I collagen to three doses of calcium delivered in milk. J. Nutr. Sci. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Choi, H.R.; Kim, S.W.; Kim, B.S.; Won, C.W.; Kim, S.Y. Association between bone mineral density and sleep duration in the Korean elderly population. Korean J. Fam. Med. 2014, 35, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Hankinson, S.E.; Schernhammer, E.S. Nightshift work and fracture risk: The Nurses’ Health Study. Osteoporos. Int. 2009, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, I.; Zuniga, A.M. Low bone mineral density in rotating-shift workers. J. Clin. Densitom. 2010, 13, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Choi, Y.J.; Chung, Y.S. Other than daytime working is associated with lower bone mineral density: The Korea National Health and Nutrition Examination Survey 2009. Calcif. Tissue Int. 2013, 93, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Pedersen, S.B.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: A randomized placebo-controlled trial. Clin. Endocrinol. 2016, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Gunn, C.A.; Weber, J.L.; McGill, A.T.; Kruger, M.C. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients 2015, 7, 2499–2517. [Google Scholar]
- Tousen, Y.; Wolber, F.M.; Chua, W.H.; Tadaishi, M.; Ishimi, Y.; Kruger, M.C. Effect of daidzein and kiwifruit on bone mineral density and equol production in ovariectomised rats. Int. J. Food Sci. Nutr. 2014, 65, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Wolber, F.M.; Tadaishi, M.; Tousen, Y.; Ishimi, Y.; Kruger, M.C. Effect of kiwifruit on bone resorption in ovariectomized mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2015, 61, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Totman, J.J.; (National University of Singapore, Singapore). Personal communication, 2016.
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.E.; Golden, N.H. Bone Health in Adolescence. Adolesc. Med. State Art. Rev. 2015, 26, 291–325. [Google Scholar] [PubMed]
- Mafi Golchin, M.; Heidari, L.; Ghaderian, S.M.; Akhavan-Niaki, H. Osteoporosis: A silent disease with complex genetic contribution. J. Genet. Genom. 2016, 43, 49–61. [Google Scholar] [CrossRef] [PubMed]
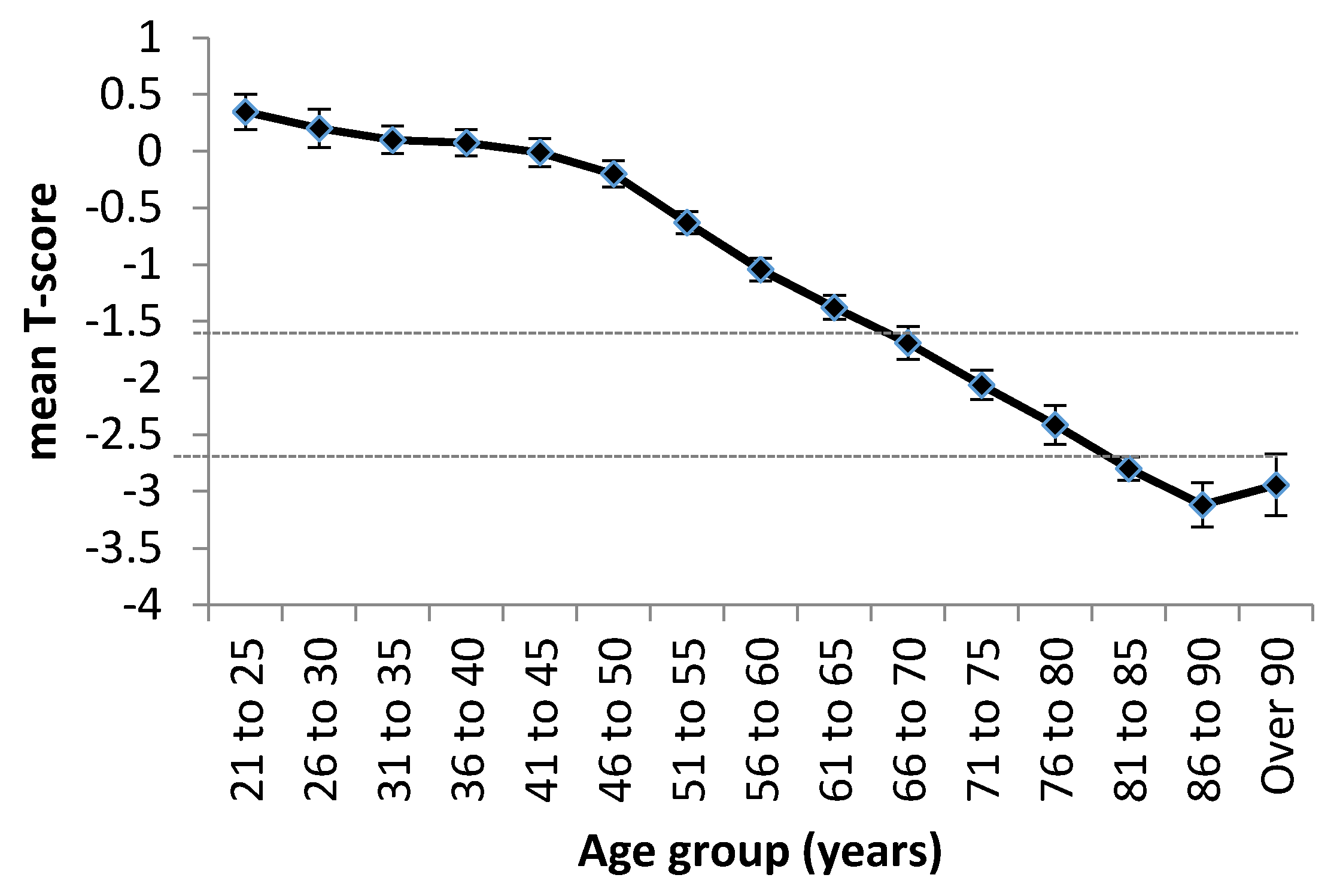

| Fat Mass | Fat Mass | Fat Mass Adjusted | Lean Mass | Lean Mass | Lean Mass Adjusted | |
|---|---|---|---|---|---|---|
| β | p | R2 | β | p | R2 | |
| Femoral Neck BMD | ||||||
| Model 1 | 0.46 | <0.001 | 0.20 | 0.51 | <0.001 | 0.26 |
| Model 2 | 0.39 | <0.001 | 0.35 | 0.49 | <0.001 | 0.40 |
| Spine BMD | ||||||
| Model 1 | 0.44 | <0.001 | 0.19 | 0.51 | <0.001 | 0.26 |
| Model 2 | 0.38 | <0.001 | 0.25 | 0.48 | <0.001 | 0.30 |
| Hip BMD | ||||||
| Model 1 | 0.54 | <0.001 | 0.29 | 0.58 | <0.001 | 0.33 |
| Model 2 | 0.50 | <0.001 | 0.36 | 0.59 | <0.001 | 0.40 |
| Fracture risk | ||||||
| Model 1 | −0.24 | 0.001 | 0.05 | −0.31 | <0.001 | 0.09 |
| Model 2 | −0.16 | 0.05 | 0.15 | −0.19 | 0.04 | 0.15 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruger, M.C.; Wolber, F.M. Osteoporosis: Modern Paradigms for Last Century’s Bones. Nutrients 2016, 8, 376. https://doi.org/10.3390/nu8060376
Kruger MC, Wolber FM. Osteoporosis: Modern Paradigms for Last Century’s Bones. Nutrients. 2016; 8(6):376. https://doi.org/10.3390/nu8060376
Chicago/Turabian StyleKruger, Marlena C., and Frances M. Wolber. 2016. "Osteoporosis: Modern Paradigms for Last Century’s Bones" Nutrients 8, no. 6: 376. https://doi.org/10.3390/nu8060376





