Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study †
Abstract
:1. Introduction
2. Materials and Methods
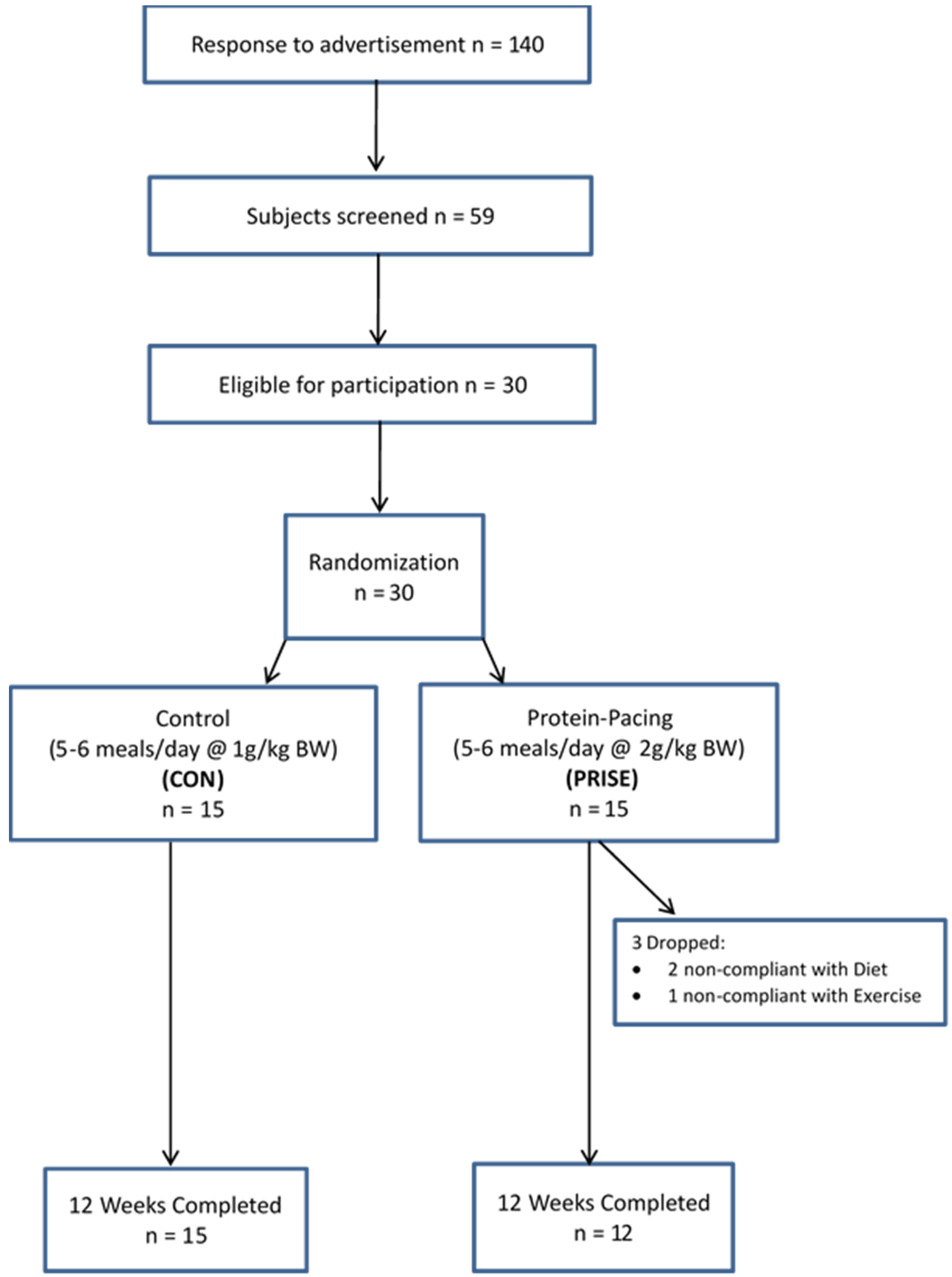
2.1. Participants
2.2. Experimental Design
Study Timeline
2.3. Nutrition Intervention
2.4. RISE Exercise Training Protocol
2.5. Laboratory Testing Procedures
2.6. Cardiometabolic Biomarkers
2.7. Resting Energy Expenditure (REE)
2.8. Total and Regional Body Composition
2.9. Dietary Intake and Feelings of Hunger and Satiety
2.10. Physical Performance Assessments
2.11. Statistical Analysis
3. Results
3.1. Participant Characteristics and Compliance
3.2. Muscular Fitness and Exercise Performance
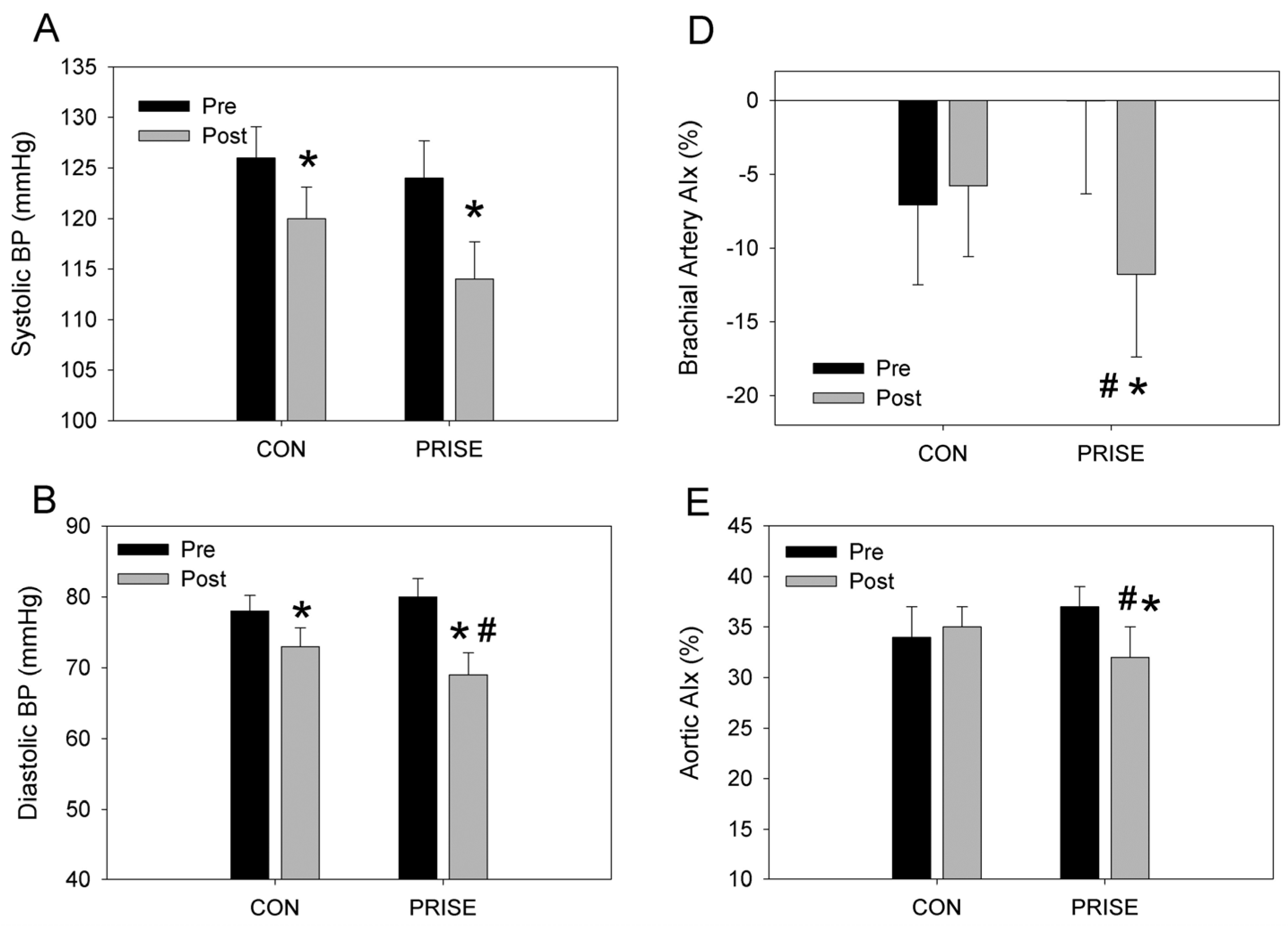
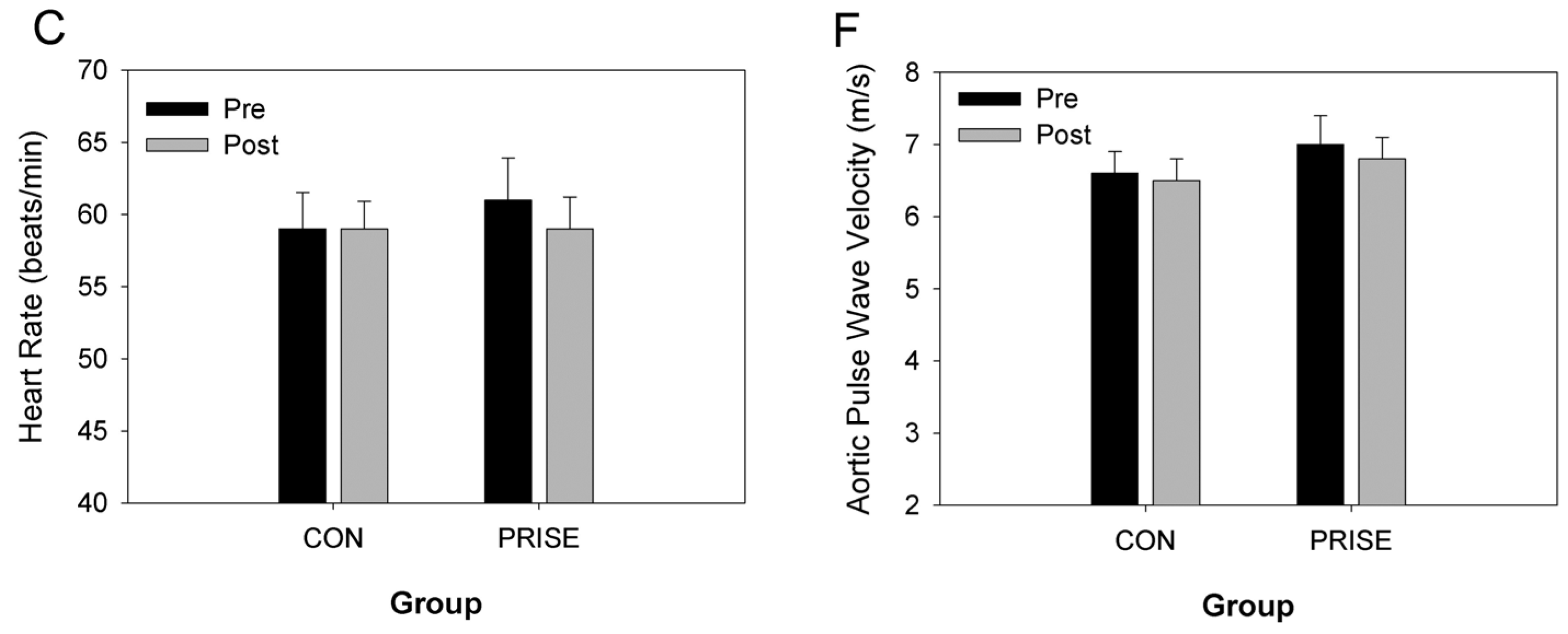
3.3. Cardiovascular Health
3.4. Body Composition
3.5. Diet, Satiety, and Hunger
3.6. Metabolic Profile
4. Discussion
4.1. Fitness and Performance Outcomes
4.2. Cardiovascular Health
4.3. Body Composition
4.4. Hunger Ratings and Dietary Intake
4.5. Metabolic Profile
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PRISE | protein-pacing, resistance, interval, stretching, endurance training |
| CON | control |
| AIx, | augmentation index |
| PWV | pulse wave velocity |
| SUs | sit-ups |
| PUs, | push-ups |
| 5-km TT | five kilometer time-trial |
| BT’s | bench throws |
| JS’s | jump squats |
| DBP | diastolic blood pressure |
| SBP | systolic blood pressure |
| TC | total cholesterol |
| HDL-C | high density lipoprotein cholesterol; |
| LDL-C | low density lipoprotein cholesterol |
| TRGs | triglycerides |
| GLU | glucose |
| REE | resting energy expenditure |
References
- Arciero, P.J.; Ormsbee, M.J.; Gentile, C.L.; Nindl, B.C.; Brestoff, J.R.; Ruby, M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity 2013, 21, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Baur, D.; Connelly, S.; Ormsbee, M.J. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: The prise study. J. Appl. Physiol. 2014, 117, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Volk, B.M.; Gomez, A.L.; Kunces, L.J.; Kupchak, B.R.; Freidenreich, D.J.; Aristizabal, J.C.; Saenz, C.; Dunn-Lewis, C.; Ballard, K.D.; et al. Whey protein supplementation during resistance training augments lean body mass. J. Am. Coll. Nutr. 2013, 32, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.D.; Taylor, L.W.; Outlaw, J.; Williams, L.; Campbell, B.; Foster, C.A.; Smith-Ryan, A.; Urbina, S.; Hayward, S. The effects of pre- and post-exercise whey vs. Casein protein consumption on body composition and performance measures in collegiate female athletes. J. Sports Sci. Med. 2013, 12, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Miller, V.J.; Ward, E. Performance enhancing diets and the prise protocol to optimize athletic performance. J. Nutr. Metab. 2015, 2015, 715859. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Gentile, C.L.; Martin-Pressman, R.; Ormsbee, M.J.; Everett, M.; Zwicky, L.; Steele, C.A. Increased dietary protein and combined high intensity aerobic and resistance exercise improves body fat distribution and cardiovascular risk factors. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 373–392. [Google Scholar] [PubMed]
- Glowacki, S.P.; Martin, S.E.; Maurer, A.; Baek, W.; Green, J.S.; Crouse, S.F. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med. Sci. Sports Exerc. 2004, 36, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.J.; Syrotuik, D.; Martin, T.P.; Burnham, R.; Quinney, H.A. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur. J. Appl. Physiol. 2000, 81, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Cantarow, M.E.; Livermore, A.A.; McEntee, K.B.; Brown, L.S. Differences in the use of protein supplements and protein-rich food as seen among us recreational athletes. Top. Clin. Nutr. 2015, 30, 167–173. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Marriott, B.P.; Williams, C.; Judelson, D.A.; Glickman, E.L.; Geiselman, P.J.; Dotson, L.; Mahoney, C.R. Patterns of dietary supplement use among college students. Clin. Nutr. 2015, 34, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.; Alaranta, A.; Helenius, I.; Vasankari, T. Dietary supplementation habits and perceptions of supplement use among elite finnish athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 271–279. [Google Scholar] [PubMed]
- Moore, D.R.; Soeters, P.B. The biological value of protein. Nestle Nutr. Inst. Workshop Ser. 2015, 82, 39–51. [Google Scholar] [PubMed]
- Arentson-Lantz, E.; Clairmont, S.; Paddon-Jones, D.; Tremblay, A.; Elango, R. Protein: A nutrient in focus. Appl. Physiol. Nutr. Metab. 2015, 40, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Murphy, C.H.; Longland, T.M.; Phillips, S.M. Role of protein and amino acids in promoting lean mass accretion with resistance exercise and attenuating lean mass loss during energy deficit in humans. Amino Acids 2013, 45, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44 (Suppl. 1), S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Hartman, J.W.; Wilkinson, S.B. Dietary protein to support anabolism with resistance exercise in young men. J. Am. Coll. Nutr. 2005, 24, 134s–139s. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport 2010, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.B.; Rybalka, E.; Stathis, C.G.; Cribb, P.J.; Hayes, A. Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. J. Int. Soc. Sports Nutr. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Bangsbo, J.; Jensen, J.; Bibby, B.M.; Madsen, K. Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Kies, A.K.; Jeukendrup, A.E.; Tipton, K.D. Effect of increased dietary protein on tolerance to intensified training. Med. Sci. Sports Exerc. 2011, 43, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Vegge, G.; Ronnestad, B.R.; Ellefsen, S. Improved cycling performance with ingestion of hydrolyzed marine protein depends on performance level. J. Int. Soc. Sports Nutr. 2012, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. A brief review of higher dietary protein diets in weight loss: A focus on athletes. Sports Med. 2014, 44, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Rakobowchuk, M.; Tanguay, S.; Burgomaster, K.A.; Howarth, K.R.; Gibala, M.J.; MacDonald, M.J. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans. Am. J. Physiol. 2008, 295, R236–R242. [Google Scholar] [CrossRef] [PubMed]
- Sivasankaran, S.; Pollard-Quintner, S.; Sachdeva, R.; Pugeda, J.; Hoq, S.M.; Zarich, S.W. The effect of a six-week program of yoga and meditation on brachial artery reactivity: Do psychosocial interventions affect vascular tone? Clin. Cardiol. 2006, 29, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Combined aerobic and resistance training and vascular function: Effect of aerobic exercise before and after resistance training. J. Appl. Physiol. 2007, 103, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Park, S.Y.; Seo, D.Y.; Sanchez-Gonzalez, M.A.; Baek, Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011, 18, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.A.; Givens, D.I.; Lovegrove, J.A. The impact of milk proteins and peptides on blood pressure and vascular function: A review of evidence from human intervention studies. Nutr. Res. Rev. 2013, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Kupchak, B.R.; Volk, B.M.; Mah, E.; Shkreta, A.; Liptak, C.; Ptolemy, A.S.; Kellogg, M.S.; Bruno, R.S.; Seip, R.L.; et al. Acute effects of ingestion of a novel whey-derived extract on vascular endothelial function in overweight, middle-aged men and women. Br. J. Nutr. 2013, 109, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Bastiaans, K.; Keogh, J.B. High protein diets decrease total and abdominal fat and improve cvd risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. NMCD 2009, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Lassnig, E.; Berent, R.; Eber, B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004, 109, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, M. Effects of resistance training on arterial stiffness: A meta-analysis. Br. J. Sports Med. 2013, 47, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Gentile, C.L.; Pressman, R.; Everett, M.; Ormsbee, M.J.; Martin, J.; Santamore, J.; Gorman, L.; Fehling, P.C.; Vukovich, M.D.; et al. Moderate protein intake improves total and regional body composition and insulin sensitivity in overweight adults. Metabolism 2008, 57, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Kim, J.E.; Amankwaah, A.F.; Gordon, S.L.; Weinheimer-Haus, E.M. Higher total protein intake and change in total protein intake affect body composition but not metabolic syndrome indexes in middle-aged overweight and obese adults who perform resistance and aerobic exercise for 36 weeks. J. Nutr. 2015, 145, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Orris, S.; Scheiner, M.; Gonzalez, A.; Peacock, C.A. A high protein diet (3.4 g/kg/day) combined with a heavy resistance training program improves body composition in healthy trained men and women—A follow-up investigation. J. Int. Soc. Sports Nutr. 2015, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Peacock, C.A.; Ellerbroek, A.; Fromhoff, B.; Silver, T. The effects of consuming a high protein diet (4.4 g/kg/day) on body composition in resistance-trained individuals. J. Int. Soc. Sports Nutr. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie-Shalders, K.L.; Byrne, N.M.; Slater, G.J.; King, N.A. The effect of a whey protein supplement dose on satiety and food intake in resistance training athletes. Appetite 2015, 92, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Ward, E.; Holst, J.J.; Astrup, A.; Ormsbee, M.J.; Connelly, S.; Arciero, P.J. Resistant starch and protein intake enhances fat oxidation and feelings of fullness in lean and overweight/obese women. Nutr. J. 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front. Endocrinol. 2014, 5, 162. [Google Scholar]
- Rossi, F.E.; Fortaleza, A.C.; Neves, L.M.; Buonani, C.; Picolo, M.R.; Diniz, T.A.; Kalva-Filho, C.A.; Papoti, M.; Lira, F.S.; Freitas Junior, I.F. Combined training (aerobic plus strength) potentiates a reduction in body fat but demonstrates no difference on the lipid profile in postmenopausal women when compared to aerobic training with a similar training load. J. Strength Cond. Res. 2015. [Google Scholar] [CrossRef] [PubMed]

| CON (n = 15) | PRISE (n = 12) | |
|---|---|---|
| Age (year) | 42 ± 7 | 42 ± 9 |
| Height (cm) | 166 ± 6 | 165 ± 7 |
| Weight (kg) | 65 ± 9 | 65 ± 7 |
| Body Mass Index (kg/m2) | 24 ± 3 | 24 ± 2 |
| Systolic Blood Pressure (mmHg) | 126 ± 11 | 127 ± 18 |
| Diastolic Blood Pressure (mmHg) | 78 ± 8 | 82 ± 12 |
| Pulse Pressure (mmHg) | 48 ± 6 | 44 ± 6 |
| Heart Rate (beats/min) | 59 ± 11 | 61 ± 7 |
| Total Cholesterol (mg/dL) | 184 ± 22 | 185 ± 37 |
| HDL Cholesterol (mg/dL) | 68 ± 17 | 67 ± 11 |
| LDL Cholesterol (mg/dL) | 97 ± 21 | 107 ± 25 |
| Triglycerides (mg/dL) | 88 ± 44 | 88 ± 43 |
| Glucose (mg/dL) | 81 ± 7 | 81 ± 6 |
| Pre | Post | ||
|---|---|---|---|
| Body Weight (kg) | CON | 65.4 ± 9.4 | 64.8 ± 9.5 |
| PRISE | 64.8 ± 7.3 | 64.6 ± 7.3 | |
| Body Fat (%) * | CON | 31.9 ± 6.6 | 30.9 ± 6.2 |
| PRISE | 30.8 ± 6.1 | 29.5 ± 7.0 | |
| Fat Mass (kg) * | CON | 20.3 ± 6.3 | 19.6 ± 6.1 |
| PRISE | 19.3 ± 5.4 | 18.6 ± 5.9 | |
| Fat Free Mass (kg) * | CON | 44.8 ± 5.2 | 45.2 ± 5.1 |
| PRISE | 45.0 ± 4.1 | 45.9 ± 4.3 | |
| Lean Body Mass (kg) * | CON | 42.3 ± 5.0 | 42.7 ± 4.8 |
| PRISE | 42.6 ± 4.0 | 43.5 ± 4.2 | |
| % Lean Body Mass (%) * | CON | 65.2 ± 6.7 | 66.5 ± 5.9 |
| PRISE | 66.1 ± 5.6 | 67.7 ± 6.2 | |
| Abdominal Fat (%) * | CON | 30.8 ± 10.6 | 29.0 ± 10.0 |
| PRISE | 28.5 ± 9.5 | 26.7 ± 11.5 | |
| Hip Fat (%) * | CON | 35.9 ± 5.6 | 34.7 ± 5.1 |
| PRISE | 35.9 ± 6.4 | 33.7 ± 7.2 | |
| Pre | Post | ||
|---|---|---|---|
| Caloric Intake (kcal/day) | CON | 1631 ± 285 | 1608 ± 282 |
| PRISE | 1662 ± 149 | 1756 ± 171 | |
| Fat Intake (g/day) | CON | 58 ± 15 | 56 ± 20 |
| PRISE | 58 ± 17 | 53 ± 19 | |
| Carbohydrate Intake (g/day) | CON | 188 ± 55 | 193 ± 53 |
| PRISE | 172 ± 63 | 177 ± 42 | |
| Protein Intake (g/day) # | CON | 77 ± 12 | 69 ± 10 |
| PRISE | 75 ± 23 | 131 ± 16 | |
| Protein Intake (g/kg BW/day) # | CON | 1.2 ± 0.2 | 1.1 ± 0.1 |
| PRISE | 1.2 ± 0.4 | 2.0 ± 0.1 | |
| Cholesterol Intake (mg/day) # | CON | 212 ± 115 | 169 ± 99 |
| PRISE | 170 ± 139 | 286 ± 125 | |
| Sodium Intake (mg/day) | CON | 1856 ± 920 | 1993 ± 639 |
| PRISE | 1816 ± 594 | 1822 ± 620 | |
| Fiber Intake (g/day) | CON | 21 ± 7 | 27 ± 11 |
| PRISE | 19 ± 7 | 23 ± 8 | |
| How hungry are you feeling? (0–100) | CON | 40 ± 17 | 45 ± 17 |
| PRISE | 42 ± 22 | 44 ± 23 | |
| How full do you feel? (0–100) | CON | 28 ± 18 | 35 ± 15 |
| PRISE | 24 ± 21 | 34 ± 23 | |
| How much food could you eat? (0–100) # | CON | 44 ± 11 | 54 ± 10 |
| PRISE | 49 ± 20 | 43 ± 19 | |
| What is your desire to eat? (0–100) | CON | 42 ± 14 | 47 ± 17 |
| PRISE | 41 ± 33 | 43 ± 27 | |
| Pre | Post | ||
|---|---|---|---|
| Resting Metabolic Rate (kcal/day) * | CON | 1385 ± 195 | 1322 ± 147 |
| PRISE | 1453 ± 147 | 1367 ± 98 | |
| Respiratory Exchange Ratio | CON | 0.80 ± 0.04 | 0.80 ± 0.05 |
| PRISE | 0.80 ± 0.05 | 0.79 ± 0.04 | |
| CHOox (%) | CON | 34 ± 20 | 33 ± 17 |
| PRISE | 32 ± 16 | 30 ± 14 | |
| FATox (%) | CON | 66 ± 20 | 67 ± 17 |
| PRISE | 68 ± 16 | 70 ± 14 | |
| Fasting Blood Glucose (mg/dL) * | CON | 81 ± 7 | 83 ± 5 |
| PRISE | 81 ± 6 | 84 ± 6 | |
| Insulin (uU/mL) | CON | 2.7 ± 1.2 | 2.5 ± 0.5 |
| PRISE | 2.5 ± 0.4 | 2.5 ± 0.4 | |
| Total Cholesterol (mg/dL) * | CON | 185 ± 22 | 182 ± 21 |
| PRISE | 185 ± 37 | 175 ± 27 | |
| HDL Cholesterol (mg/dL) | CON | 68 ± 17 | 69 ± 13 |
| PRISE | 67 ± 11 | 67 ± 12 | |
| LDL Cholesterol (mg/dL) | CON | 93 ± 21 | 94 ± 25 |
| PRISE | 107 ± 25 | 96 ± 27 | |
| Total Cholesterol/HDL | CON | 2.9 ± 0.9 | 2.7 ± 0.6 |
| PRISE | 2.6 ± 0.4 | 2.6 ± 0.3 | |
| Triglycerides (mg/dL) | CON | 92 ± 40 | 87 ± 31 |
| PRISE | 88 ± 43 | 89 ± 28 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciero, P.J.; Ives, S.J.; Norton, C.; Escudero, D.; Minicucci, O.; O’Brien, G.; Paul, M.; Ormsbee, M.J.; Miller, V.; Sheridan, C.; et al. Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study. Nutrients 2016, 8, 332. https://doi.org/10.3390/nu8060332
Arciero PJ, Ives SJ, Norton C, Escudero D, Minicucci O, O’Brien G, Paul M, Ormsbee MJ, Miller V, Sheridan C, et al. Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study. Nutrients. 2016; 8(6):332. https://doi.org/10.3390/nu8060332
Chicago/Turabian StyleArciero, Paul J., Stephen J. Ives, Chelsea Norton, Daniela Escudero, Olivia Minicucci, Gabe O’Brien, Maia Paul, Michael J. Ormsbee, Vincent Miller, Caitlin Sheridan, and et al. 2016. "Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study" Nutrients 8, no. 6: 332. https://doi.org/10.3390/nu8060332
APA StyleArciero, P. J., Ives, S. J., Norton, C., Escudero, D., Minicucci, O., O’Brien, G., Paul, M., Ormsbee, M. J., Miller, V., Sheridan, C., & He, F. (2016). Protein-Pacing and Multi-Component Exercise Training Improves Physical Performance Outcomes in Exercise-Trained Women: The PRISE 3 Study. Nutrients, 8(6), 332. https://doi.org/10.3390/nu8060332








