1. Introduction
The beneficial effect of Se supplementation for men’s health is still a controversial issue [
1,
2,
3,
4]. Our Se supplementation (200 μg/day as selenized yeast for six months) studies from Auckland, New Zealand indicated that beneficial effects of Se vary considerably among individuals. Using baseline and post-supplementation related biomarker changes of this group; we have reported that benefits of Se are influenced by demographic, lifestyle and health factors, as well as with gene polymorphisms in the seleno-genome [
5,
6,
7]. Our studies also indicated that Se supplementation increased the H
2O
2-induced DNA damage in 56.6% of New Zealand men while the erythrocyte thioredoxin reductase (TR) activity was increased in 60% of this population [
5,
6]. Cassidy
et al. have shown that the electrophile bound TR can disrupt p53 confirmation leading to caspase activation [
8]. Therefore, it is interesting to know whether increased TR activity and the H
2O
2-induced DNA damage observed with Se supplementation have any association with the homeostatic caspase-cleaved apoptosis levels in these men.
Apoptosis is proposed to be part of the beneficial effects of Se supplementation [
9] and is a parameter worth assessing in Se supplementation studies. Among the main cytoskeletal proteins in epithelial cells is the cytokeratine 18 (CK18) [
10]. Various caspases cleave intermediate filaments of these proteins during apoptosis. Cleavage of CK18 is said to occur at aspartic acid residue 396 [
11] and is detectable with the M30 monoclonal antibody [
11]. This cleavage is initiated by caspase 9 and followed by the action of caspases 3 and 7 [
12].
Imbalances in protein and carbohydrate consumption have also been shown to affect DNA fragmentation in rat models [
13]. The influence of saturated and polyunsaturated fatty acid dietary intakes with DNA adduct formation has also been reported before [
14,
15,
16]. Meanwhile, the mechanism of action on DNA integrity related to Se is reportedly influenced by other micro-nutrient co-factors, including Vitamin B6, B12, zinc, folate, methionine, betaine and choline [
17,
18,
19,
20].
We present here an initial hypothesis-generating analysis to identify possible reasons associated with the extreme increases and decreases of H
2O
2-induced DNA damage recorded from peripheral blood leukocytes of men after six months of Se supplementation. Data reviewed include demographic, lifestyle and medication factors, biomarkers and the single nucleotide polymorphisms in antioxidant genotypes between these subgroups [
6,
7]. Additionally, we assess the influence of dietary and activity levels and caspase-cleaved K18 (CCK18) levels measured in plasma between these extreme subgroups. Testing of the hypothesis generated was carried out using correlation between biomarkers and dietary intake in a complete dataset.
4. Discussion
Our current analysis shows that the extremes of the H
2O
2-induced DNA damage are not associated with the demographic, lifestyle, and medication factors. There were no significant variations among the % energy contribution from major nutrients or % of fats as saturated, poly-unsaturated and mono-unsaturated components, or intake of Se, Vitamin B12, Vitamin B6 or zinc. However, dietary components of folate and methionine were marginally but significantly different between the two groups. These two nutrients are known for their one carbon metabolism that provides methyl groups for various methyl transfer reactions [
21]. Methionine is an essential amino acid providing methyl groups for methylation of bio-molecules including nucleic acids [
22].The process of methyl transfer is co-supported by folate, and both are ubiquitously found in all living cells [
21]. Therefore, any imbalance in these nutrients can have a profound influence on DNA integrity and methylation reactions [
21]. The requirement of folate in maintaining DNA stability is thought to be due to its ability to prevent excessive uracil incorporation to DNA [
23,
24]. Group B records a lower dietary intake of folate compared to the recommendation of 400 μg/day specified by the Australian National Health and Medical Research Council and the New Zealand Ministry of Health (ANHMRC and NZMOH), while Group A records a level above this requirement [
25]. However, there was no indication of a correlation between dietary folate intake and H
2O
2-induced DNA damage with the complete dataset at both baseline and post-supplementation time points. Correlation between fresh blood DNA damage and dietary folate intake for the complete dataset at baseline however showed a non-significant negative trend, which is lost after Se supplementation. Dietary methionine, however, showed significant negative correlation with H
2O
2-induced DNA damage post-supplementation with the complete dataset.
The ANHMRC and NZMOH have not specified a methionine nutritional requirement on its own, although they have discussed the influence of other nutrients including cysteine and folate in maintaining methionine levels [
25]. According to the World Health Organisation standards, dietary requirement of the sulphur amino acid methionine on its own is 10.4 mg·kg
−1·day
−1 [
26]. The median body weight of Group A was 78 kg which gives an estimated methionine requirement of 0.81 g per day. The median body weight of Group B was 75 kg which gives an estimated methionine requirement of 0.78 g per day. Group A recorded a dietary methionine level 7.4% below the estimated requirement, while Group B recorded a level 39.7% above the estimated requirement. Methionine supplemented diets have previously shown 122% increase of micronuclei frequency in peripheral blood without any increases of these marker in heart and liver tissue of adult female mice [
27]. Rodents supplemented with 3 fold higher L-methionine levels compared to controls have shown mitochondrial ROS generation and oxidative damage to mitochondrial DNA in liver tissue [
28]. Meanwhile, a 40% methionine restricted diet has shown a decrease in mitochondrial ROS production, reduced damage in mitochondrial DNA and decreased methylation in genomic DNA in rat liver tissue [
29,
30]. As methionine is the ultimate source of methyl groups in DNA methylation reactions, it is known that dietary methionine intake can control methylation reactions in mammals [
31]. Waterland has also reviewed methionine supplementation effects on S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH), the latter of which is converted from the former by the DNA methyltransferase reactions. According to Waterland, there exists a complex variation in ultimate SAM to SAH ratios based on the age of animals as well as based on other nutrient co-factors provided through the supplemented diet. Our subgroup data suggests that men in Group B have overcome folate deficiency and methionine excess by supplemented Se, while those with sufficient levels of folate and relatively lower levels of methionine showed detrimental effects from Se supplementation. Ingested selenomethionine (SeMet) can either get directly incorporated to proteins instead of methionine or metabolised to other low molecular weight selenium containing molecules such as selenohomocysteine and selenocysteine. The latter happens through a methionine cycle and a trans-sulfuration pathway as described by Lazard
et al. [
32]. Lazard
et al. have further confirmed production of superoxide radicals by SeMet treatment in mutant yeast strains. They also discuss ready oxidation of low molecular weight metabolites of SeMet such as selenocysteine and selenohomocysteine as the basis of superoxide radical formation, rather than being direct effects of SeMet. The organic Se supplement SelPlex has approximately 34%–36% of low molecular weight seleno molecules apart from having 63% of SeMet [
33,
34]. These low molecular weight seleno molecules too could have the potential of getting oxidised to form superoxide and other free radicals. Increased H
2O
2-induced DNA damage displayed by individuals in Group A after Se supplementation could be due to such free radical effects of SelPlex metabolites although the majority of participants from the complete study cohort have not recorded symptomatic toxicities [
6]. However, higher methionine levels in Group B individuals seem to have supressed SeMet induced free radical formation and subsequent DNA damage. Meanwhile, it has been reported that the ribosomes fail to distinguish between methionine and SeMet loaded tRNA during the process of translation [
35,
36]. This makes it possible for SeMet to modulate the effects of excess methionine by competing for its translation into proteins. On the other hand, Lazard
et al. have recorded the efficacy of methionine (0–40 μM) in suppressing SeMet (2.5–20.0 μm) related growth impacts in
Saccharomyces cerevisiae [
32]. It may well be that both methionine and SeMet can modulate damaging effects of one another. If folate is sufficiently available, that could support recycling of methionine from homocysteine or SeMet from selenohomocysteine using folate dependent methionine synthase [
32,
37]. This could reduce the chances of free radical formation in the process of homocysteine or selenohomocysteine oxidation. Therefore, low folate levels in Group B have shown higher DNA damage at baseline as recycling of endogenous homocysteine and selenohomocysteine would have been impaired.
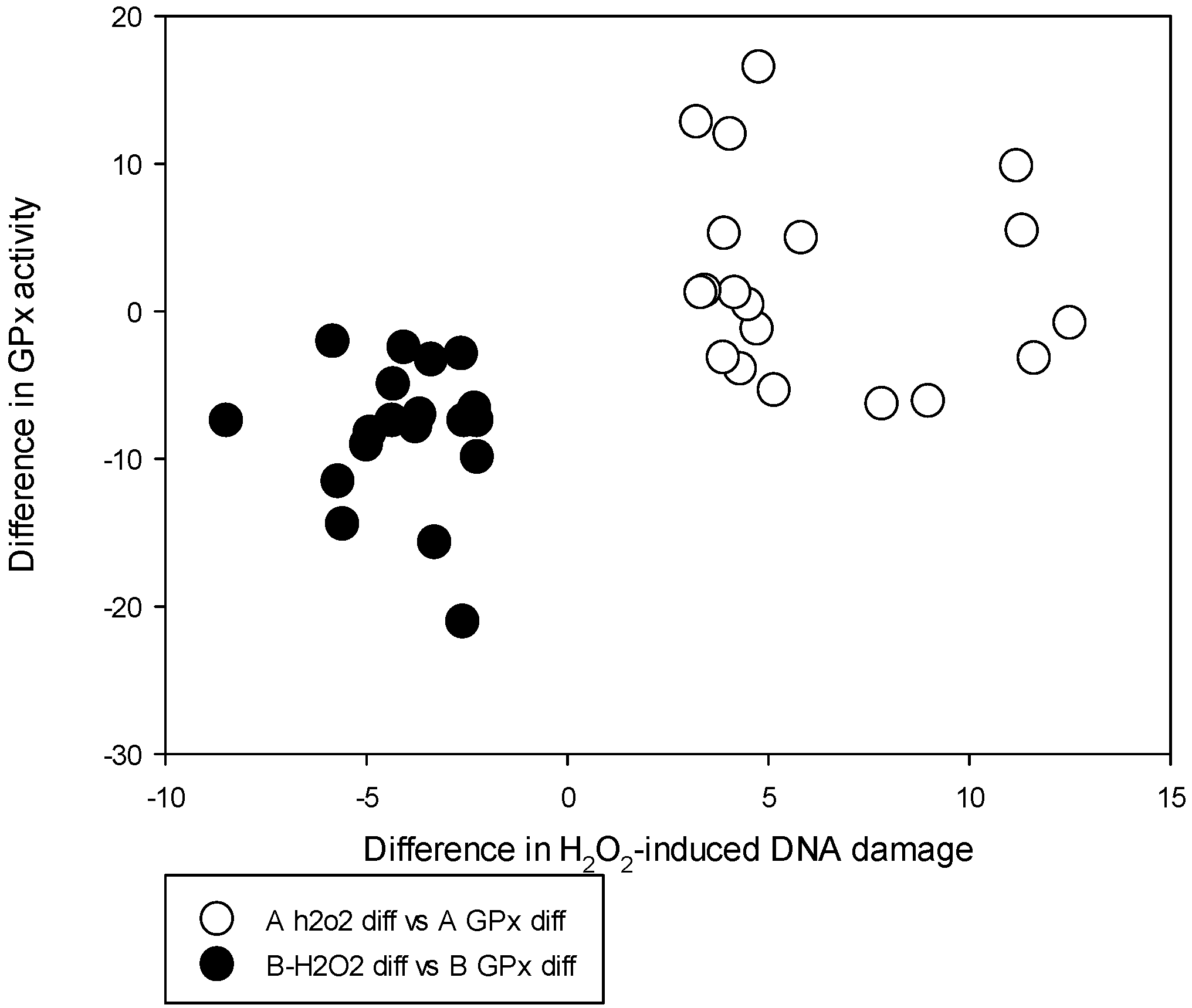
The significant decrease in the GPx activity corresponding to decreased H
2O
2-induced DNA damage post-supplementation in Group B is similar to our previous recording of supplementation effects shown by 141 men from the highest baseline tertile of H
2O
2-induced DNA damage [
6]. The increase in the fresh blood DNA damage among Group A and a decrease in Group B after supplementation are similar to supplementation variation recorded with the highest and lowest baseline fresh blood DNA damage tertiles recorded from 424 men from our previous studies [
6]. These data indicate that the biomarker data of this small group of 38 men are representative of the dataset from our original cohort of men that showed a variation of DNA damage with Se supplementation. A review of known GPxs by Brigelius-Flohe and Maiorino records that the phospholipid hydroperoxide glutathione peroxidase (GPx4) is an important modulator of apoptosis [
38]. In particular, the 12, 15 lipoxygenase (LOX)-induced apoptosis caused by activation of the apoptosis inducing factor (AIF) instead of caspase activation is shown to be modulated by GPx4 [
38]. Previous studies indicate that, depending on Se availability, the function of different GPxs can interchange their leading roles Using chimeric constructs having mutually exchanged 3′UTR regions in GPx1, GPx4 and GPx2 coding regions, it has been shown that both GPx4 and GPx1 activities can get overexpressed under Se adequate conditions but not the GPx2 [
39]. Our GPx activity records were from erythrocyte lysates which measure only the cytosolic GPx1 activity, and we cannot prove that the reduction of GPx activity in Group B is compensated by activation of GPx4 and GPx2.
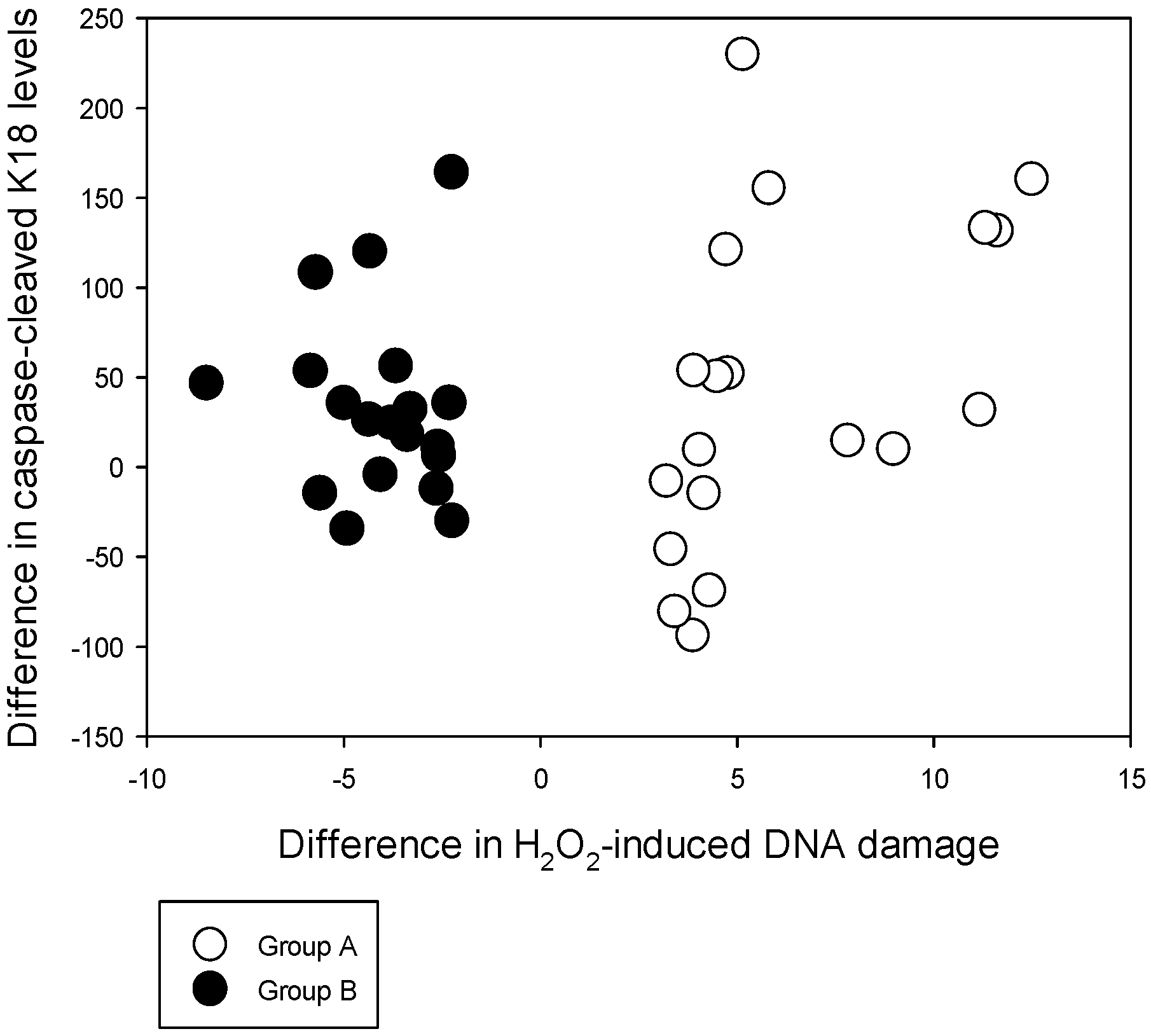
Men in both extreme subgroups show a significant (~25%) increase in the CCK18 levels with Se supplementation suggesting an increase of homeostatic caspase-induced apoptosis. It has been proposed that health benefits of Se could well be due to its apoptotic effects [
9,
40]. Effects of Se on apoptosis is recorded in
in vitro experiments with various cell lines including SWO-38 human glioma cells, A549/DDP human lung adenocarcinoma cells, HL-60 leukaemia cell, a549 human lung cancer cells, MCF-7 human breast adenocarcinoma cells, normal human skin fibroblasts, cervico-uterine cancer cells and prostate cancer cells. These experiments have used a variety of inorganic and organic Se supplements including sodium selenite, SeMet, modified Se nanoparticles, methylseleninic acid, Se-containing phycocyanin (Se-PC) and 22-oxo-26-selenocyanocholestane [
41,
42,
43,
44,
45,
46,
47,
48,
49]. Hawkes
et al. have carried out a Se supplementation study with 16 healthy men for 1 year with 300 μg/day of high selenized yeast or placebo. Whole blood gene expression with DNA micro-array data from these men before and after Se supplementation has shown that the main functional pathway affected by Se supplementation was that of FAS apoptosis signalling [
50]. The magnitude of CCK18 increase in our cohort is comparable with such increases recorded after Docetaxal treatment on hormone refractory prostate cancer patients (~20% CCK18 level increase) [
51]. With cultured Synoviocytes, it has been shown that the caspases-8 and 9 (apoptosis initiator caspases) and caspase-3 (apoptosis executioner caspase) are upregulated in folate-deprived conditions compared to folate sufficient conditions [
52]. These authors have further shown that folate deprivation was associated with a two fold increase in reactive oxygen species (ROS) compared to that of the folate supplemented medium. Using a rat model treated with the chemotherapeutic agent Cisplatin, Bodiga
et al. have shown that those supplemented with folate show an attenuation of Cisplatin induced apoptosis in the intestinal epithelium. They have shown this decrease using M30 staining, DNA fragmentation, and caspase-3 activity [
53]. With our data, the extremes of H
2O
2-induced DNA damage cannot be significantly associated with variable changes in the caspase-cleaved apoptosis levels. However, those with lower dietary folate and higher dietary methionine (Group B) could have been exposed to higher caspase-cleaved apoptosis at pre-supplementation compared to Group A, which subsequently produced a non-significant increase in the CCK18 levels. Therefore, our results could be indicating that Se supplementation produces a homeostatic increase in apoptosis while modulating subsequent lower folate and higher methionine related effects on leukocyte DNA integrity.
The concentrations of Se that up-regulated the CCK18 levels in plasma in Groups A and B were within the limits recorded in Zhao
et al. study for up regulating apoptosis [
48]. Se supplementation with selenite on a cell line derived from fish has shown both apoptotic and necrotic effects beyond a level of 10 μM. Levels above 10 μM also induced mitochondrial membrane potential damage, DNA damage and elevated production of reactive oxygen species considered to be associated with cell death [
54]. Using two malignant mesothelioma cell sublines, Nilsonne
et al. have shown that apoptosis occurs between 5 and 30 μM selenite. The mean post-supplementation serum Se levels in groups A and B in our study were 177.3 ng/mL (2.25 μM/L) and 175.7 ng/mL (2.23 μM/L) respectively. These are below the 10 μM limit of Se from selenite reported in Selvaraj
et al. [
54] for fish cell lines or the levels reported from human tumour cell lines associated with apoptosis [
55]. However, accumulation of Se in tissues other than in serum could have been different. Stoebe
et al. have recorded that Se supplementation in farmed deer increased Se levels and the GPx activity in tissue at varying degrees [
56]. Brozmanova
et al. have reviewed varying mechanisms involved in apoptotic processes between different seleno-compounds [
57]. Therefore, for organic Se supplements, the limit required for apoptosis could be different to that of selenite.
Although we have measured GPx activity in the erythrocyte lysates, DNA damage was measured in the leukocytes. GPx1 is the lowest in the hierarchy of known selenoproteins. Such selenoproteins are known to give way to those on top of the hierarchy (e.g., GPx4) in times of selenium deficiency [
38]. As the primary outcome of our original study was to optimize Se benefits, we had to work out levels associated with optimizing the GPx1 activity. This can be measured in either erythrocyte or leukocyte lysates [
58]. A study carried out with GPx activity measurements in horses reveals that levels from both erythrocytes and leukocytes are comparable among control horses, while among horses with a respiratory distress, the erythrocyte levels were higher than that of the leukocytes [
58]. Erythrocyte and granulocyte GPx activity is known to be comparable in humans although lymphocytes record several fold higher levels [
59]. As our DNA damage assessment protocol was with the general leukocyte population, it is represented by a higher proportion of granulocytes than lymphocytes [
60]. In addition, red blood cells (RBC) have a definitive lifespan of approximately 120 days [
61,
62] while that of leukocytes varies [
63,
64]. As our supplementation time was six calendar months or approximately 180 days, we needed to assess supplementation effects in blood cells born within the supplementation period and survived through our assessment; and RBCs were the ideal candidate for that. Unfortunately, as RBCs in circulation lack a nucleus, we had no option but to select leukocytes for our DNA damage assessments.
We acknowledge several shortcomings in our study. Supplementation was carried out with two batches of SelPlex supplements, as the duration of recruitment and supplementation required a longer time than originally expected. Although SelPlex generally has 63% SeMet and 34%–36% of other low molecular weight seleno molecules, possible variation between the batches was unavoidable. We have not recorded key intermediate molecules of the methionine cycle and trans-sulfuration pathway. We have based our folate and methionine nutrition status based on dietary pattern given by a four day diet diary, which may not be an adequate measure to represent dietary intake within six months. Besides, supplementation would have changed the methionine profiles in blood. However, we do not have folate and methionine levels measured in blood at baseline and post-supplementation time points to strengthen our argument. We recommend that these molecules be assessed in future Se supplementation related studies.