Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparations of Carthamus Tinctorius (CT)
2.2. Animals and Experimental Protocols
2.3. Indirect Measurement of Blood Pressure in Conscious Rats
2.4. Hemodynamic Measurement
2.5. Isolated Aortic Rings Protocols
2.6. Perfusion Mesenteric Vascular Beds Protocols
2.7. Assay of Vascular O2− Production
2.8. Assay of Plasma Malondialdehyde (MDA)
2.9. Assay of Plasma Ang II and Plasma Nitric Oxide Metabolites (NOx)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
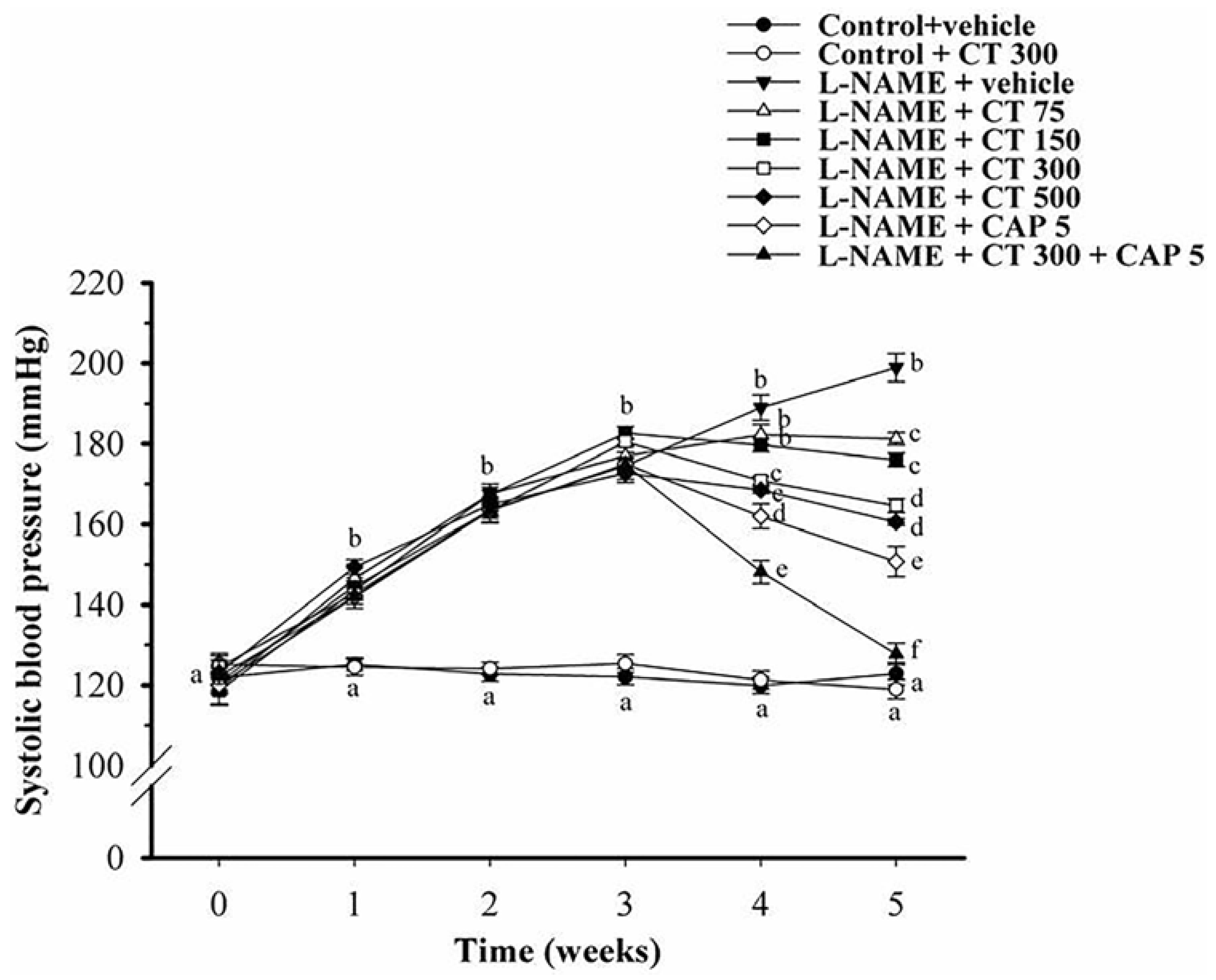
3.1. Effects of CT Extract and Captopril Supplementation on Systolic Blood Pressure in Conscious Rats
3.2. Effects of CT Extract and Captopril Supplementation on Hemodynamic Parameters
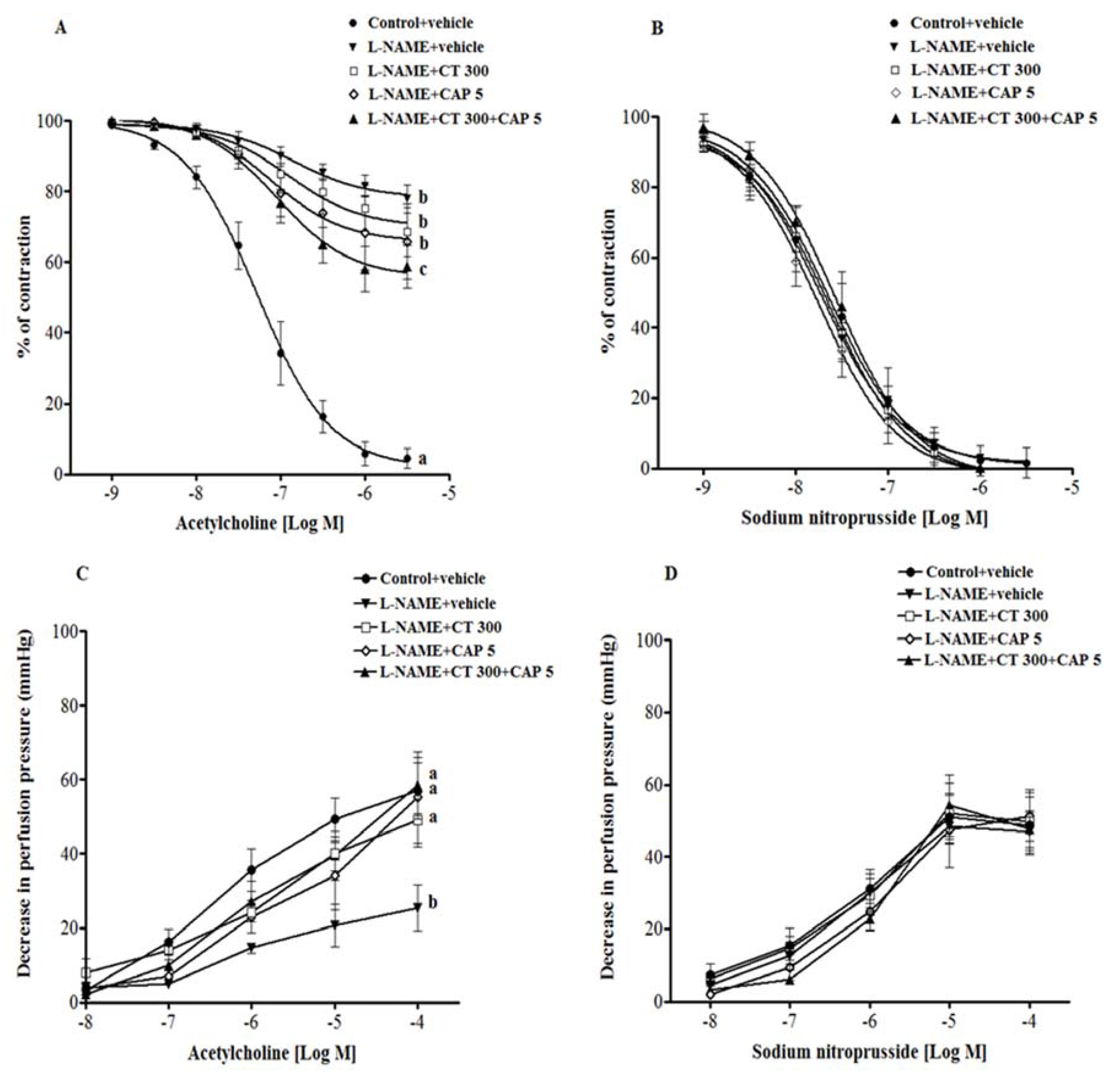
3.3. Effects of CT Extract and Captopril Supplementation on Vascular Reactivity in Aortic Rings and Mesenteric Vascular Beds
3.4. Effects of CT Extract and Captopril Supplementation on Oxidative Stress Status in l-NAME Hypertensive Rats
3.5. Effects of CT Extract and Captopril Supplementation on Plasma Ang II and Plasma NOx Levels
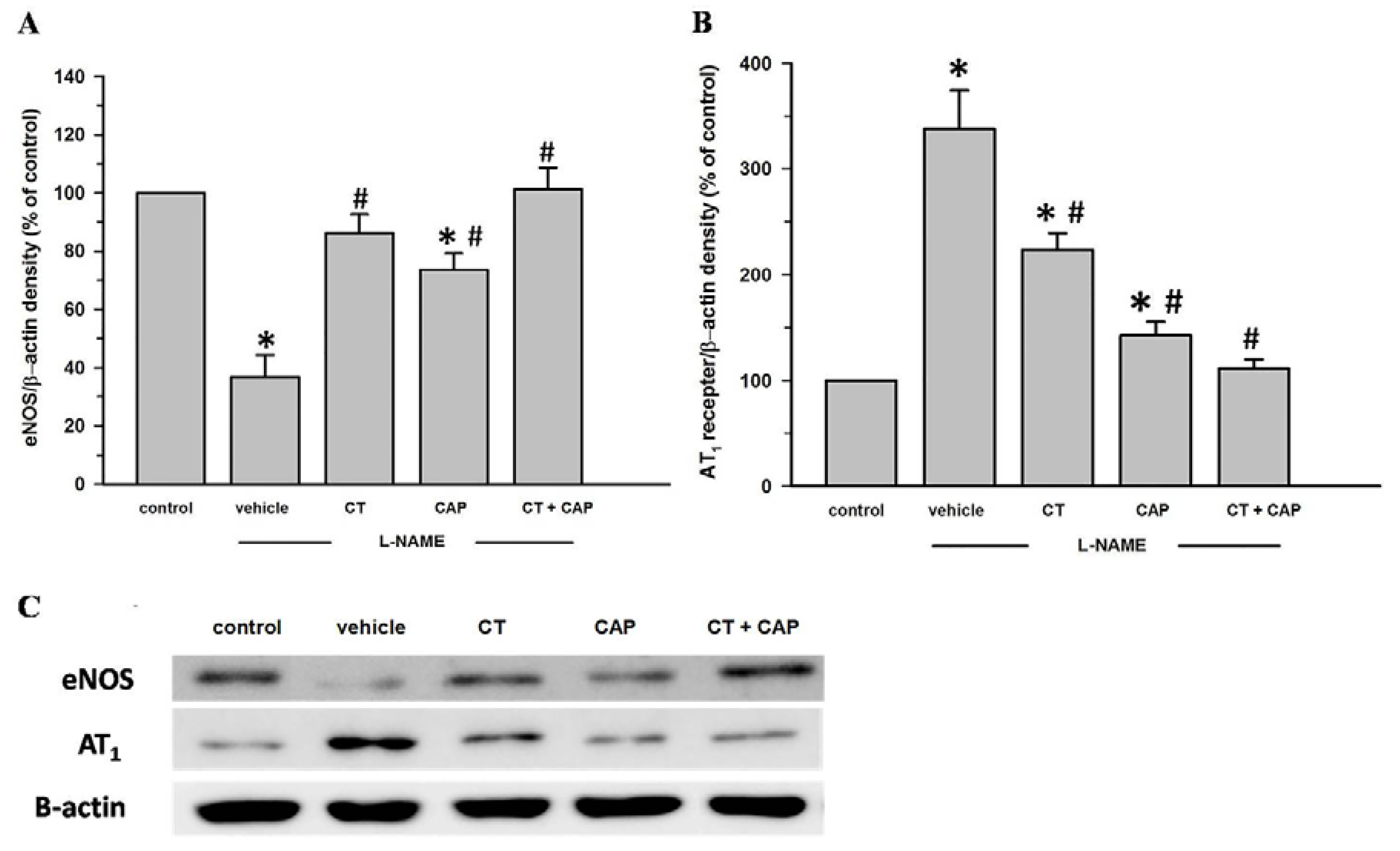
3.6. Effects of CT Extract and Captopril Supplementation on Aortic eNOS and AT1R Protein Expression in l-NAME Hypertensive Rats
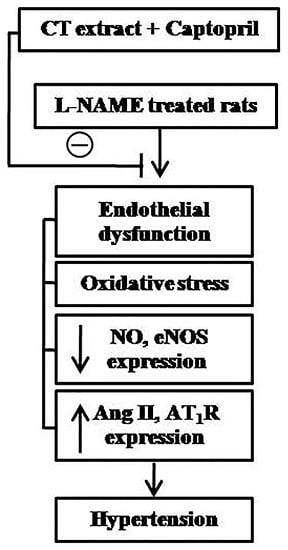
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Ribeiro, M.O.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Prachaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Welbat, J.U.; Pakdeechote, P. Asiatic acid alleviates cardiovascular remodeling in rats with l-NAME-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I.; Pechanova, O.; Kristek, F. Mechanism of structural remodelling of the rat aorta during long-term NG-nitro-l-arginine methyl ester treatment. Jpn. J. Pharmacol. 1999, 81, 99–106. [Google Scholar] [PubMed]
- Pakdeechote, P.; Prachaney, P.; Berkban, W.; Kukongviriyapan, U.; Kukongviriyapan, V.; Khrisanapant, W.; Phirawatthakul, Y. Vascular and antioxidant effects of an aqueous mentha cordifolia extract in experimental NG-nitro-l-arginine methyl ester-induced hypertension. Z. Naturforsch. C 2014, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Raja, B. Syringic acid ameliorates l-NAME-induced hypertension by reducing oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.; Ahmad, M.; Al-Shabanah, O.A.; Raza, M. Effects of losartan on blood pressure, oxidative stress, and nitrate/nitrite levels in the nitric oxide deficient hypertensive rats. Recept. Channels 2004, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rincon, J.; Correia, D.; Arcaya, J.L.; Finol, E.; Fernandez, A.; Perez, M.; Yaguas, K.; Talavera, E.; Chavez, M.; Summer, R.; et al. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015, 124, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Metsarinne, K.; Tikkanen, I. Role of angiotensin ii in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J. Hum. Hypertens. 1995, 9, S19–S24. [Google Scholar] [PubMed]
- Zanchi, A.; Schaad, N.C.; Osterheld, M.C.; Grouzmann, E.; Nussberger, J.; Brunner, H.R.; Waeber, B. Effects of chronic no synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system. Am. J. Physiol. 1995, 268, H2267–H2273. [Google Scholar] [PubMed]
- Rivera-Jardon, F.F.; Castro-Moreno, P.; Figueroa-Guillen, E.S.; Gallardo-Ortiz, I.A.; Godinez-Hernandez, D.; Ibarra-Barajas, M. Angiotensin II augments renal vasoconstriction via At1 receptors in l-NAME-induced hypertensive rats. Proc. West Pharmacol. Soc. 2009, 52, 47–49. [Google Scholar] [PubMed]
- Melaragno, M.G.; Fink, G.D. Role of ang II in hypertension produced by chronic inhibition of nitric oxide synthase in conscious rats. Am. J. Physiol. 1996, 271, H806–H811. [Google Scholar] [PubMed]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane nadh/nadph oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Simkova, M.; Kovacs, L. The ace inhibitor, captopril, in the light of new clinical studies. Ceska Slov Farm 2002, 51, 63–67. [Google Scholar] [PubMed]
- Mak, I.T.; Freedman, A.M.; Dickens, B.F.; Weglicki, W.B. Protective effects of sulfhydryl-containing angiotensin converting enzyme inhibitors against free radical injury in endothelial cells. Biochem. Pharmacol. 1990, 40, 2169–2175. [Google Scholar] [CrossRef]
- Singh, N.P.; Uppal, M.; Anuradha, S.; Agarwal, A.; Rizvi, S.N. Angiotensin converting enzyme inhibitors and cough—A north indian study. J. Assoc. Phys. India 1998, 46, 448–451. [Google Scholar]
- Li, H.X.; Han, S.Y.; Wang, X.W.; Ma, X.; Zhang, K.; Wang, L.; Ma, Z.Z.; Tu, P.F. Effect of the carthamins yellow from Carthamus tinctorius L. On hemorheological disorders of blood stasis in rats. Food Chem. Toxicol. 2009, 47, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. In traditional chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.M.; He, J.; Jiang, J.S.; Chen, Z.; Yang, Y.N.; Zhang, P.C. NMR solution structure study of the representative component hydroxysafflor yellow A and other quinochalcone C-glycosides from Carthamus tinctorius. J. Nat. Prod. 2013, 76, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Li, H.X.; Ma, X.; Zhang, K.; Ma, Z.Z.; Tu, P.F. Protective effects of purified safflower extract on myocardial ischemia in vivo and in vitro. Phytomedicine 2009, 16, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wei, Y.; Yang, X.Z.; Li, F.G.; Hu, J.; Cheng, R.F. Hypotensive effects of safflower yellow in spontaneously hypertensive rats and influence on plasma renin activity and angiotensin II level. Yao Xue Xue Bao 1992, 27, 785–787. [Google Scholar] [PubMed]
- Lu, F.J.; Lin, J.T.; Wang, H.P.; Huang, W.C. A simple, sensitive, non-stimulated photon counting system for detection of superoxide anion in whole blood. Experientia 1996, 52, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. The 1991 ulf von euler lecture. The l-arginine: Nitric oxide pathway. Acta Physiol. Scand. 1992, 145, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Qian, L.B.; Zhu, L.G.; Liang, H.T.; Tan, Y.N.; Lu, H.T.; Lu, J.F.; Wang, H.P.; Xia, Q. Betulinic acid ameliorates endothelium-dependent relaxation in l-NAME-induced hypertensive rats by reducing oxidative stress. Eur. J. Pharm. Sci. 2011, 44, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Pakdeechote, P.; Kukongviriyapan, U.; Prachaney, P.; Kukongviriyapan, V. Asiatic acid reduces blood pressure by enhancing nitric oxide bioavailability with modulation of enos and p47phox expression in l-NAME-induced hypertensive rats. Phytother. Res. 2014, 28, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Landino, L.M.; Crews, B.C.; Timmons, M.D.; Morrow, J.D.; Marnett, L.J. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in phenolic composition and antioxidant activity during flower development of safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Li, H.X.; Bai, C.C.; Wang, L.; Tu, P.F. Component analysis and free radical-scavenging potential of panax notoginseng and Carthamus tinctorius extracts. Chem. Biodivers. 2010, 7, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.C.; Fortepiani, L.A.; Ruiz-Marcos, F.M.; Atucha, N.M.; Garcia-Estan, J. Role of at1 receptors in the renal papillary effects of acute and chronic nitric oxide inhibition. Am. J. Physiol. 1998, 274, R760–R766. [Google Scholar] [PubMed]
- Natalin, H.M.; Garcia, A.F.; Ramalho, L.N.; Restini, C.B. Resveratrol improves vasoprotective effects of captopril on aortic remodeling and fibrosis triggered by renovascular hypertension. Cardiovasc. Pathol. 2015, 25, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Macdonald, G.J.; Duggan, K.A. Effects of nitric oxide synthase inhibition on angiotensin receptors and metabolism in the pregnant hypertensive rat. Clin. Sci. 2001, 100, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Sasamura, H.; Sakamaki, Y.; Itoh, H.; Saruta, T. Developmental activity of the renin-angiotensin system during the “critical period” modulates later l-NAME-induced hypertension and renal injury. Hypertens. Res. 2007, 30, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Vascular oxidative stress upregulates angiotensin II type I receptors via mechanisms involving nuclear factor κB. Clin. Exp. Hypertens. 2014, 36, 367–373. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Control + Vehicle (n = 12) | Control + CT 300 (mg/kg/Day) (n = 8) | l-NAME + Vehicle (n = 12) | l-NAME + CT 75 (mg/kg/Day) (n = 8) | l-NAME + CT 150 (mg/kg/Day) (n = 8) | l-NAME + CT 300 (mg/kg/Day) (n = 12) | l-NAME + CT 500 (mg/kg/Day) (n = 8 ) | l-NAME + CAP 5 (mg/kg/Day) (n = 12) | l-NAME + CAP 5 + CT 300 (mg/kg/Day) (n = 12) |
|---|---|---|---|---|---|---|---|---|---|
| SP (mmHg) | 120.7 ± 1.8 a | 119.8 ± 1.7 a | 199.3 ± 3.4 b | 186.7 ± 5.7 c | 178.6 ± 3.7 c | 163.3 ± 3.2 d | 169.5 ± 2.4 c,d | 151.8 ± 3.7 e | 125.3 ± 2.6 a |
| DP (mmHg) | 77.3 ± 3.3 a | 78.6 ± 1.8 a | 140.3 ± 4.1 b | 124.28 ± 7.00 c | 109 ± 4.9 d | 106.9 ± 2.2 d | 118.46 ± 3 c,d | 87.8 ± 4.6 a | 75.8 ± 3.6 a |
| MAP (mmHg) | 91.7 ± 2.6 a | 92.3 ± 1.5 a | 160 ± 3.8 b | 145.11 ± 6.36 c | 132.2 ± 4.1 d | 125.7 ± 2.4 d,e | 135.5 ± 2.7 c,d,e | 109.1 ± 3.8 b,c,d,e,f | 92.3 ± 2.8 a |
| PP (mmHg) | 44.9 ± 3.1 a,d | 40.4 ± 1.9 a | 59 ± 1.6 b | 59 ± 2.4 b,c | 63.3 ± 1.6 b | 54.6 ± 1.8 b,c | 50.9 ± 1.5 b,c,d | 60 ± 2.1 b | 50.3 ± 3.3 c,d |
| HR (beats/min) | 362.9 ± 3.7 a,b | 341.5 ± 12.1 a | 399 ± 8.1 a,b | 362.8 ± 10 a,b | 356.8 ± 9.2 a,b | 359.9 ± 9.9 a,b | 379 ± 4.9 a,b | 365.6 ± 14.5 a,b | 349.6 ± 10.1 a |
| HBF (mL/min/100 g tissue) | 6.3 ± 0.3 a | 5.9 ± 0.2 b | 3.1 ± 0.2 b | 3.6 ± 0.3 b | 3.9 ± 0.2 b | 4.7 ± 0.2 c | 4.4 ± 0.2 c | 4.7 ± 0.2 c | 6.2 ± 0.5 a |
| HVR (mmHg/min/100 g tissue/mL) | 13.6 ± 0.9 a | 15.7 ± 0.9 a | 44.2 ± 2.4 b | 44.3 ± 6.9 b | 34.6 ± 1.9 c | 26.8 ± 1 d | 28. 4 ± 1.2 d | 23.7 ± 1.3 d | 15.6 ± 1 a |
| Parameters | Control + Vehicle (n = 8) | l-NAME + Vehicle (n = 8) | l-NAME + CT 300 (mg/kg/Day) (n = 8) | l-NAME + CAP 5 (mg/kg/Day) (n = 8) | l-NAME + CAP 5 + CT 300 (mg/kg/Day) (n = 8) |
|---|---|---|---|---|---|
| Luciginine-Enhanced Chemiluminescence (count/mg dry wt/min) | 46.8 ± 4.4 | 116 ± 8.4 * | 94.3 ± 9.8 *,# | 52.9 ± 2.4 # | 53.9 ± 4.6 # |
| MDA (µM) | 4 ± 0.5 | 9.8 ± 0.5 * | 5.4 ± 0.4 *,# | 4.8 ± 0.2 # | 4.3 ± 0.3 # |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneesai, P.; Prasarttong, P.; Bunbupha, S.; Kukongviriyapan, U.; Kukongviriyapan, V.; Tangsucharit, P.; Prachaney, P.; Pakdeechote, P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression. Nutrients 2016, 8, 122. https://doi.org/10.3390/nu8030122
Maneesai P, Prasarttong P, Bunbupha S, Kukongviriyapan U, Kukongviriyapan V, Tangsucharit P, Prachaney P, Pakdeechote P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression. Nutrients. 2016; 8(3):122. https://doi.org/10.3390/nu8030122
Chicago/Turabian StyleManeesai, Putcharawipa, Patoomporn Prasarttong, Sarawoot Bunbupha, Upa Kukongviriyapan, Veerapol Kukongviriyapan, Panot Tangsucharit, Parichat Prachaney, and Poungrat Pakdeechote. 2016. "Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression" Nutrients 8, no. 3: 122. https://doi.org/10.3390/nu8030122