Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Intervention
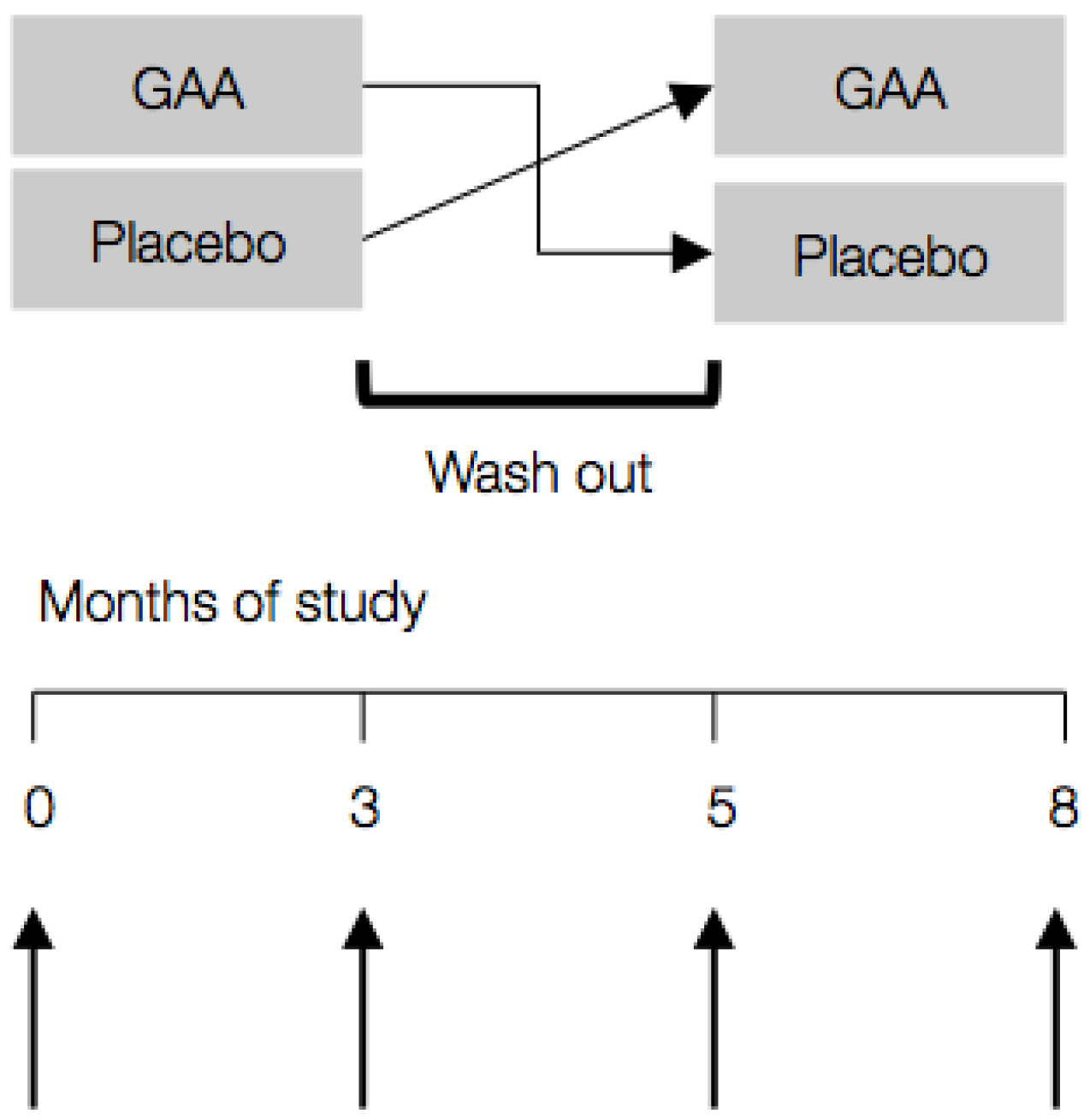
2.3. Study Protocol
2.4. Statistical Analyses
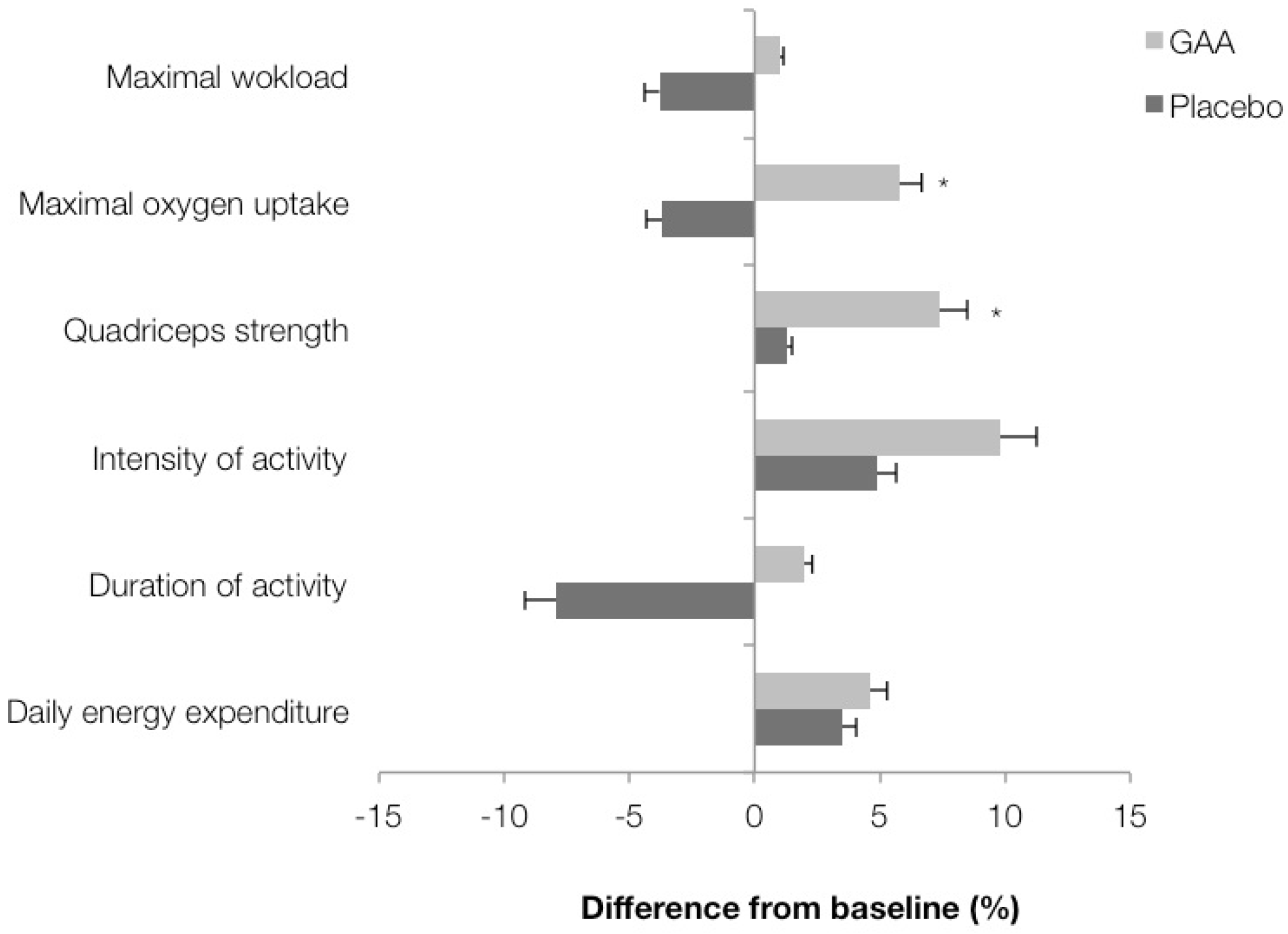
3. Results
| Baseline | At Follow up | p * | ||
|---|---|---|---|---|
| Placebo | GAA | |||
| Multidimensional fatigue score | ||||
| General fatigue | 12.1 ± 1.5 | 11.8 ± 1.5 | 11.6 ± 1.3 | 0.44 |
| Physical fatigue | 11.2 ± 1.0 | 11.6 ± 1.4 | 11.7 ± 1.2 | 0.99 |
| Reduced activity | 11.7 ± 1.6 | 13.9 ± 1.2 | 11.7 ± 1.8 | 0.00 |
| Reduced motivation | 15.2 ± 1.5 | 15.0 ± 1.8 | 13.1 ± 1.9 | 0.03 |
| Mental fatigue | 12.9 ± 1.3 | 14.0 ± 0.9 | 12.2 ± 1.7 | 0.01 |
| Musculoskeletal soreness | ||||
| At rest (score) | 1.4 ± 1.1 | 1.4 ± 1.3 | 1.2 ± 1.0 | 0.31 |
| During activity (score) | 5.0 ± 1.5 | 5.0 ± 1.8 | 4.4 ± 1.5 | 0.18 |
| Health-related quality of life | ||||
| Physical common score | 55.1 ± 4.9 | 52.8 ± 4.2 | 55.2 ± 2.8 | 0.04 |
| Mental common score | 42.4 ± 13.3 | 45.8 ± 6.5 | 51.1 ± 5.5 | 0.00 |
| Baseline | At Follow up | p * | ||
|---|---|---|---|---|
| Placebo | GAA | |||
| Serum | ||||
| GAA (µmol/L) | 3.0 ± 0.3 | 2.6 ± 0.4 | 4.2 ± 1.2 | <0.001 |
| Creatine (µmol/L) | 26.1 ± 5.1 | 35.3 ± 12.8 | 47.8 ± 13.5 | 0.048 |
| Creatinine (µmol/L) | 77.1 ± 7.8 | 77.7 ± 13.0 | 100.7 ± 14.6 | <0.001 |
| Homocysteine (µmol/L) | 9.6 ± 1.7 | 9.4 ± 1.8 | 11.9 ± 1.6 | <0.001 |
| Urine | ||||
| GAA (µmol/L) | 151.9 ± 45.2 | 146.9 ± 50.6 | 274.1 ± 101.1 | 0.004 |
| Creatine (µmol/L) | 17.6 ± 2.3 | 29.3 ± 11.2 | 42.8 ± 22.7 | 0.192 |
| Creatinine (µmol/L) | 1.0 ± 0.3 | 1.3 ± 0.4 | 1.7 ± 0.4 | 0.041 |
| Muscle | ||||
| Creatine (mmol/kg wet weight) | 27.8 ± 4.5 | 28.5 ± 4.8 | 38.0 ± 2.6 | 0.008 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CFS | Chronic fatigue syndrome |
| ESR | Erythrocyte sedimentation rate |
| GAA | Guanidinoacetic acid |
| HR | Heart rate |
| HRQL | Health-related quality of life |
| MFI | Multidimensional fatigue inventory |
| MR | magnetic resonance |
| RBC | Red blood cell count |
| VAS | Visual analog scale |
References
- Avellaneda Fernández, A.; Pérez Martín, A.; Izquierdo Martínez, M.; Arruti Bustillo, M.; Barbado Hernández, F.J.; de la Cruz Labrado, J.; Díaz-Delgado Peñas, R.; Gutiérrez Rivas, E.; Palacín Delgado, C.; Rivera Redondo, J.; et al. Chronic fatigue syndrome: Aetiology, diagnosis and treatment. BMC Psychiatry 2009, 9, S1. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Buchwald, D. Chronic fatigue syndrome: A review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Block, W.; Träber, F.; Kuhl, C.K.; Keller, E.; Lamerichs, R.; Karitzky, J.; Rink, H.; Schild, H.H. 31P-mr spectroscopy of peripheral skeletal musculature under load: Demonstration of normal energy metabolites compared with metabolic muscle diseases. Rofo 1998, 168, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Werker, C.L.; Nijhof, S.L.; van de Putte, E.M. Clinical Practice: Chronic fatigue syndrome. Eur. J. Pediatr. 2013, 172, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Brenu, E.W.; Staines, D.; Marshall-Gradisnik, S. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: A meta-analysis. Clin. Epidemiol. 2013, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Nisenbaum, R.; Hoaglin, D.C.; Unger, E.R.; Emmons, C.; Randall, B.; Stewart, J.A.; Abbey, S.; Jones, J.F.; Gantz, N.; et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch. Intern. Med. 2003, 163, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.B.; van der Meer, J.W.; Bleijenberg, G. Chronic fatigue syndrome. Lancet 2006, 367, 346–355. [Google Scholar] [CrossRef]
- Lin, J.M.; Resch, S.C.; Brimmer, D.J.; Johnson, A.; Kennedy, S.; Burstein, N.; Simon, C.J. The economic impact of chronic fatigue syndrome in Georgia: Direct and indirect costs. Cost Effect. Resour. Alloc. 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Bagnall, A.M.; Sowden, A.J.; Cornell, J.E.; Mulrow, C.D.; Ramírez, G. Interventions for the treatment and management of chronic fatigue syndrome: A systematic review. JAMA 2001, 286, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, F.M.; van der Werf, S.; Bleijenberg, G.; van der Zee, L.; van der Meer, J.W. The effect of a polynutrient supplement on fatigue and physical activity of patients with chronic fatigue syndrome: A double-blind randomized controlled trial. QJM 2002, 95, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Alraek, T.; Lee, M.S.; Choi, T.Y.; Cao, H.; Liu, J. Complementary and alternative medicine for patients with chronic fatigue syndrome: A systematic review. BMC Complement. Altern. Med. 2011, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [PubMed]
- Baker, D.H. Advances in protein-amino acid nutrition of poultry. Amino Acids 2009, 37, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [PubMed]
- Ostojic, S.M.; Niess, B.; Stojanovic, M.; Obrenovic, M. Creatine metabolism and safety profiles after six-week oral guanidinoacetic acid administration in healthy humans. Int. J. Med. Sci. 2013, 10, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010, 7, e1000251. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Garssen, B.; Bonke, B.; de Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Ferraz, M.B.; Quaresma, M.R.; Aquino, L.R.; Atra, E.; Tugwell, P.; Goldsmith, C.H. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J. Rheumatol. 1990, 17, 1022–1024. [Google Scholar] [PubMed]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.; Fernández-Solà, J.; Nuñez, E.; Fernández-Huerta, J.M.; Godás-Sieso, T.; Gomez-Gil, E. Health-related quality of life in patients with chronic fatigue syndrome: Group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1 year of follow-up. Clin. Rheumatol. 2011, 30, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Frendl, D.M.; Ware, J.E., Jr. Patient-reported functional health and well-being outcomes with drug therapy: A systematic review of randomized trials using the SF-36 health survey. Med. Care 2014, 52, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Trump, M.E.; Hanstock, C.C.; Allen, P.S.; Gheorghiu, D.; Hochachka, P.W. An (1)H-MRS evaluation of the phosphocreatine/creatine pool (tCR) in human muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R889–R896. [Google Scholar] [PubMed]
- Nacul, L.C.; Lacerda, E.M.; Pheby, D.; Campion, P.; Molokhia, M.; Fayyaz, S.; Leite, J.C.; Poland, F.; Howe, A.; Drachler, M.L. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: A repeated cross-sectional study in primary care. BMC Med. 2011, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.; Hotopf, M. Childhood predictors of self reported chronic fatigue syndrome/myalgic encephalomyelitis in adults: National birth cohort study. BMJ 2004, 329, 941. [Google Scholar] [CrossRef] [PubMed]
- Moss-Morris, R.; Deary, V.; Castell, B. Chronic fatigue syndrome. Handb. Clin. Neurol. 2013, 110, 303–314. [Google Scholar] [PubMed]
- White, P.D.; Goldsmith, K.A.; Johnson, A.L.; Potts, L.; Walwyn, R.; de Cesare, J.C.; Baber, H.L.; Burgess, M.; Clark, L.V.; Cox, D.L.; et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef]
- Yancey, J.R.; Thomas, S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Phys. 2012, 86, 741–746. [Google Scholar]
- Borsook, M.E.; Borsook, H. Treatment of cardiac decompensation with betaine and glycocyamine. Ann. West Med. Surg. 1951, 5, 830–855. [Google Scholar] [PubMed]
- Ostojic, S.M.; Stojanovic, M.D.; Hoffman, J.R. Six-week oral guanidinoacetic acid administration imporves muscular performance in healthy volunteers. J. Investig. Med. 2015, 63, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 2015, 54, 1211–1215. [Google Scholar] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. https://doi.org/10.3390/nu8020072
Ostojic SM, Stojanovic M, Drid P, Hoffman JR, Sekulic D, Zenic N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients. 2016; 8(2):72. https://doi.org/10.3390/nu8020072
Chicago/Turabian StyleOstojic, Sergej M., Marko Stojanovic, Patrik Drid, Jay R. Hoffman, Damir Sekulic, and Natasa Zenic. 2016. "Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome" Nutrients 8, no. 2: 72. https://doi.org/10.3390/nu8020072








