Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Designs
2.2. Blood Collection
2.3. Blood Analysis
2.4. Statistical Analysis
3. Results
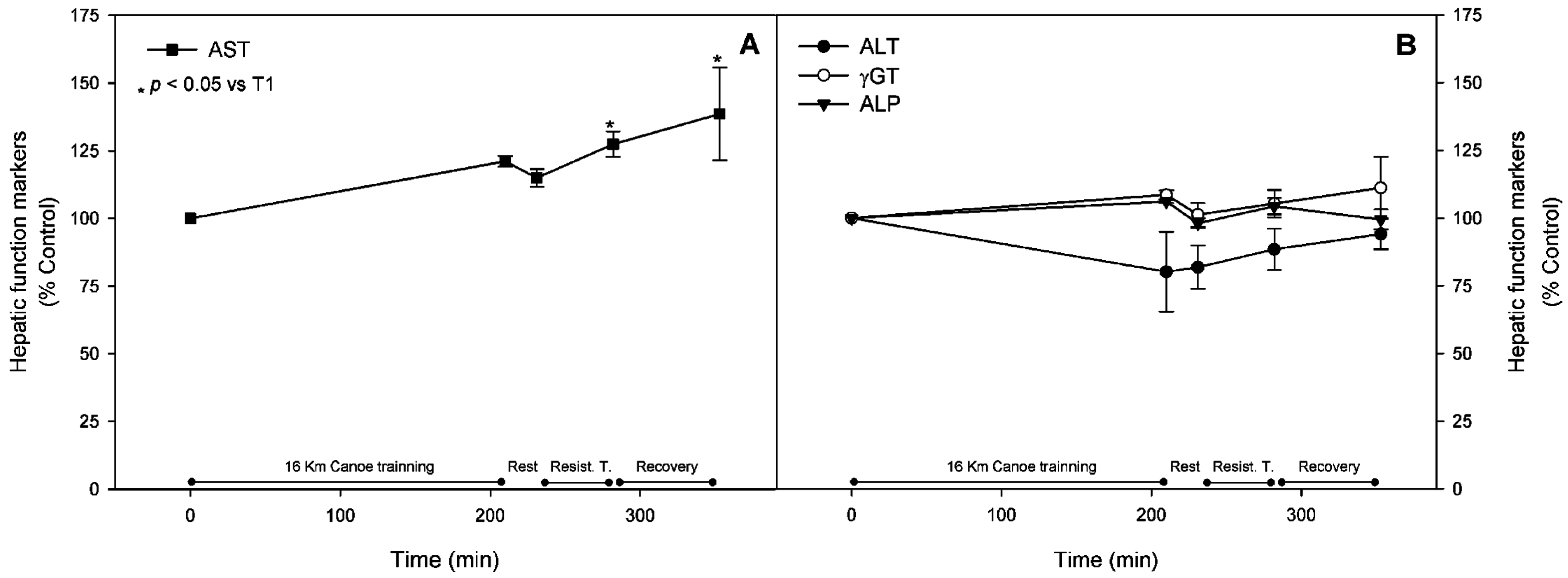
3.1. Muscle Stress Biomarkers
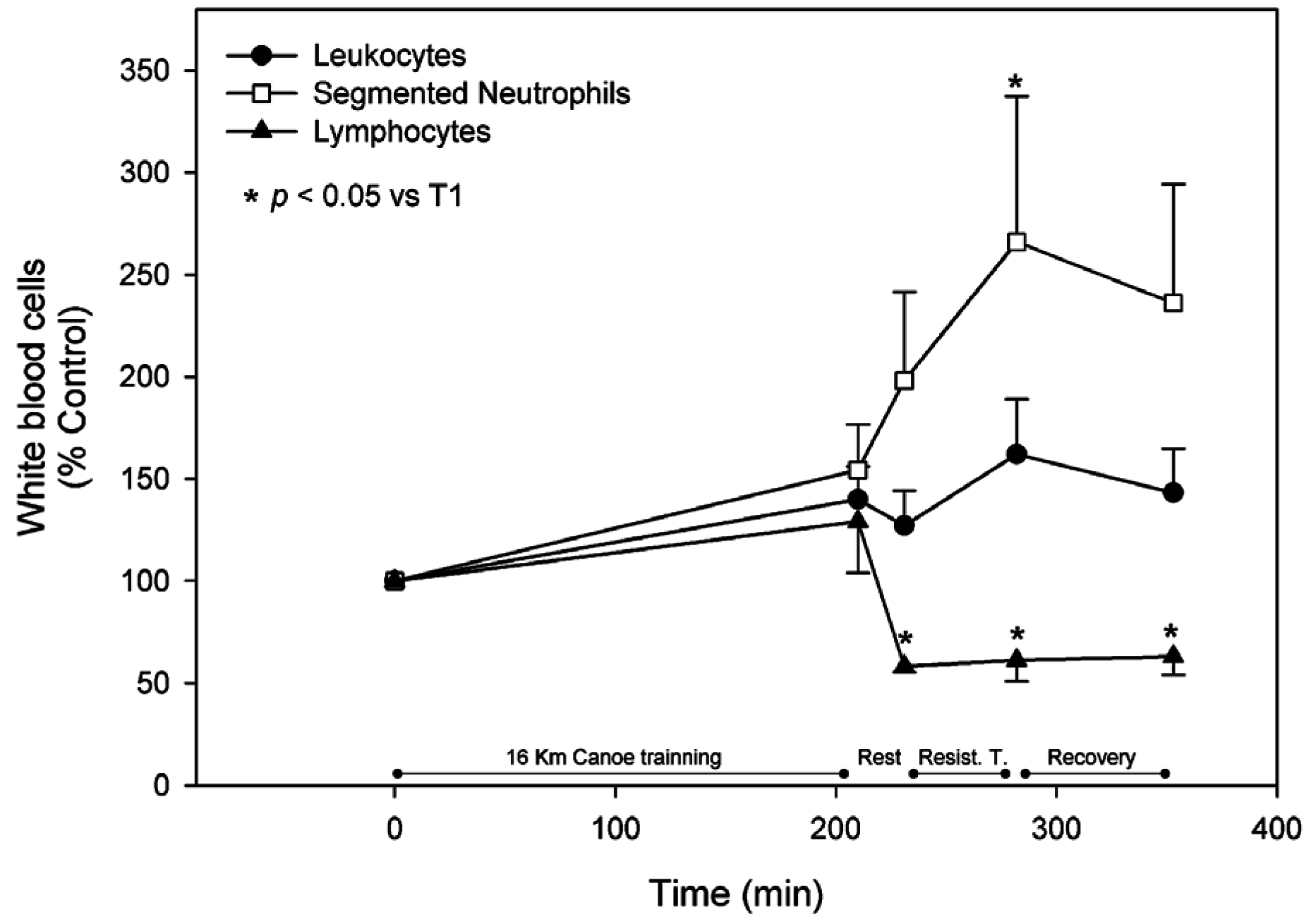
3.2. White Blood Cells
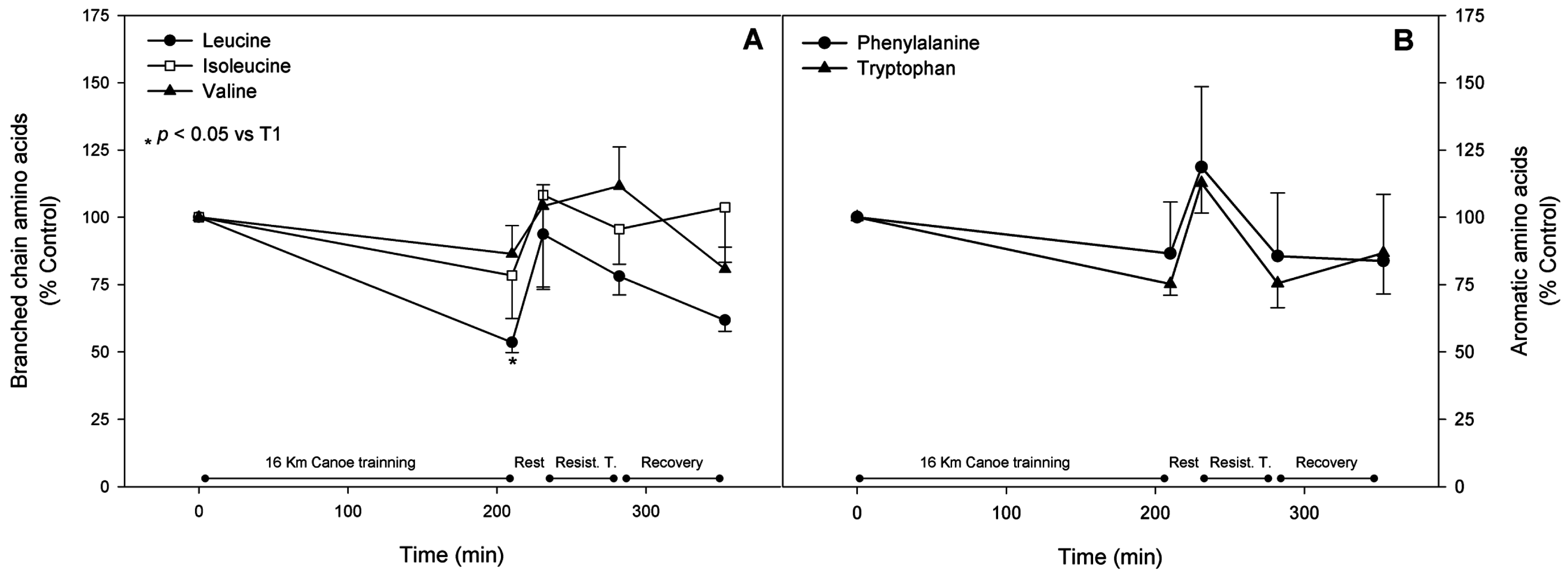
3.3. Branched Chain Amino Acids
3.4. Aromatic Amino Acids
3.5. Gluconeogenic Amino Acids
3.6. Metabolic Pathway Substrates, Intermediates, and Products
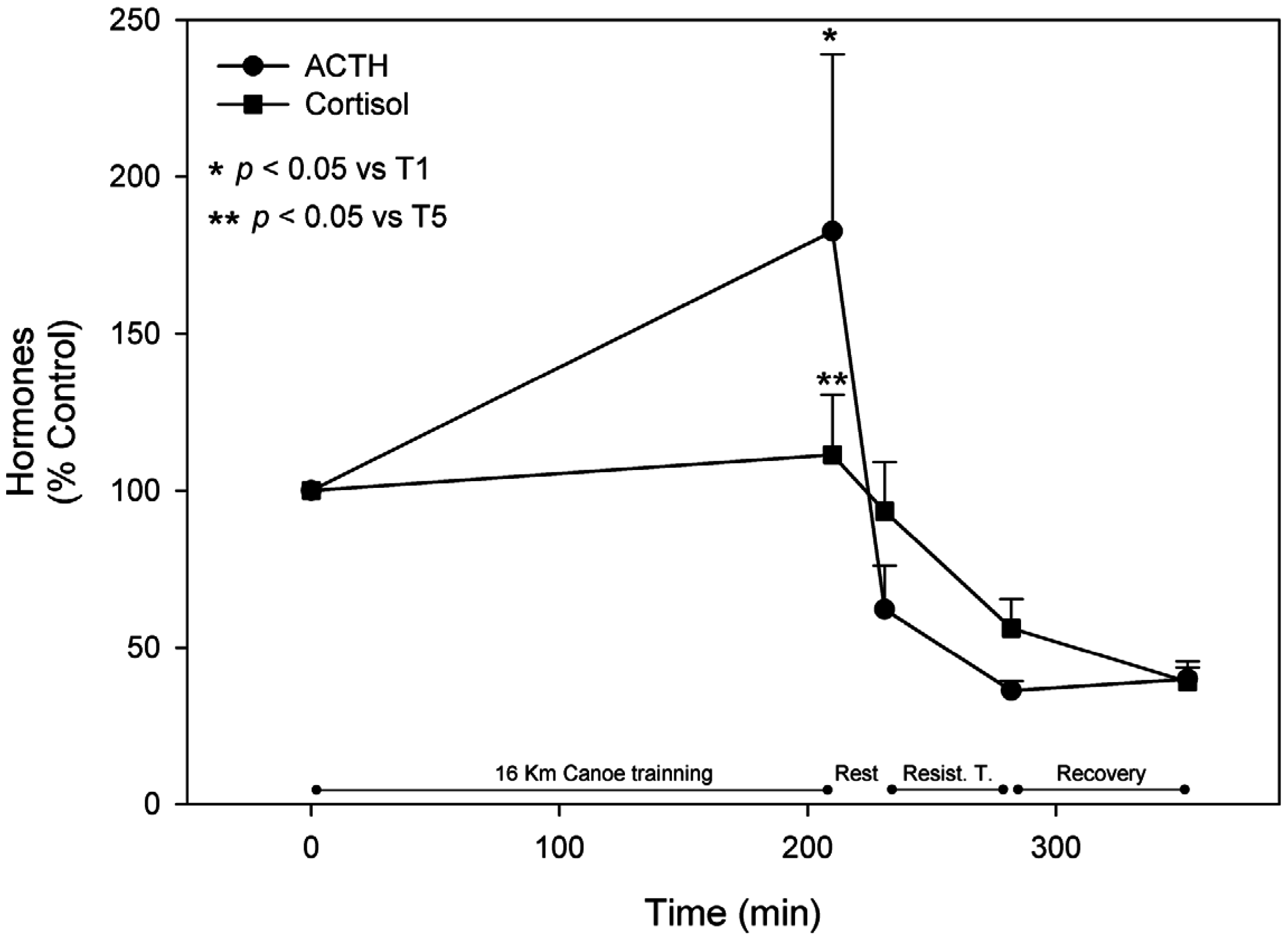
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.J.; Richter, E.A. Regulatory mechanisms of skeletal muscle protein turnover during exercise. J. Appl. Physiol. 2009, 106, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.; Nissenbaum, M.; Monteiro, A.; Gandra, P.G.; Bassini-Cameron, A.; Werneck-de-Castro, J.P.S.; Vaz de Macedo, D.; Cameron, L.-C. High-intensity ultraendurance promotes early release of muscle injury markers. Br. J. Sports Med. 2008, 42, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Tang, J.E.; Moore, D.R.; Phillips, S.M. Exercise training and protein metabolism: Influences of contraction, protein intake, and sex-based differences. J. Appl. Physiol. 2009, 106, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Bassini-Cameron, A.; Sweet, E.; Bottino, A.; Bittar, C.; Veiga, C.; Cameron, L.-C. Effect of caffeine supplementation on haematological and biochemical variables in elite soccer players under physical stress conditions. Br. J. Sports Med. 2007, 41, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Lazarim, F.L.; Antunes-Neto, J.M.F.; da Silva, F.O.C.; Nunes, L.A.S.; Bassini-Cameron, A.; Cameron, L.-C.; Alves, A.A.; Brenzikofer, R.; Vaz de Macedo, D. The upper values of plasma creatine kinase of professional soccer players during the Brazilian National Championship. J. Sci. Med. Sport 2009, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- De Souza Martins, S.C.; Romão, L.F.; Faria, G.C.; de Holanda Afonso, R.C.; Murray, S.A.; Pellizzon, C.H.; Mercer, J.A.; Cameron, L.C.; Moura-Neto, V. Effect of thyroid hormone T3 on myosin-Va expression in the central nervous system. Brain Res. 2009, 1275, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.D.; Prado, E.S.; Llosa, C.D.; Magalhães-Neto, A.; Cameron, L.C. Acute supplementation with keto analogues and amino acids in rats during resistance exercise. Br. J. Nutr. 2010, 104, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Resende, N.M.; de Magalhães Neto, A.M.; Bachini, F.; de Castro, L.E.V.; Bassini, A.; Cameron, L.C. Metabolic changes during a field experiment in a world-class windsurfing athlete: A trial with multivariate analyses. OMICS 2011, 15, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.S.; de Rezende Neto, J.M.; de Almeida, R.D.; de Melo, M.G.D.; Cameron, L.C. Keto analogue and amino acid supplementation affects the ammonaemia response during exercise under ketogenic conditions. Br. J. Nutr. 2011, 105, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.C. Mass Spectrometry Imaging: Facts and perspectives from a non-mass spectrometrist point of view. Methods 2012, 57, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.C.; Bessa, A.; Freitas-Dias, R.; Luzes, R.; Werneck-de-Castro, J.P.S.; Bassini, A.; Cameron, L.C. A sportomics strategy to analyze the ability of arginine to modulate both ammonia and lymphocyte levels in blood after high-intensity exercise. J. Int. Soc. Sports Nutr. 2012, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Bassini, A.; Magalhães-Neto, A.M.; Sweet, E.; Bottino, A.; Veiga, C.; Tozzi, M.B.; Cameron, L.C. Caffeine decreases systemic urea in elite soccer players during intermittent exercise. Med. Sci. Sports Exerc. 2013, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.A.; de Souza Dos Santos, M.C.; Rangel, I.F.; Ribeiro, M.B.; Cavalcanti-de-Albuquerque, J.P.; Ferreira, A.C.; Cameron, L.C.; Carvalho, D.P.; Werneck de Castro, J.P. Decreased serum T3 after an exercise session is independent of glucocorticoid peak. Horm. Metab. Res. 2013, 45, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Bassini, A.; Cameron, L.C. Sportomics: Building a new concept in metabolic studies and exercise science. Biochem. Biophys. Res. Commun. 2014, 445, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.A.; Speedy, D.B.; Thompson, J.M.D.; Noakes, T.D.; Mulligan, G.; Page, T.; Campbell, R.G.D.; Milne, C. Study of hematological and biochemical parameters in runners completing a standard marathon. Clin. J. Sport Med. 2004, 14, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chen, K.T.; Shee, B.W.; Chang, H.C.; Huang, Y.J.; Yang, R.S. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J. Gastroenterol. 2004, 10, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L. Nutritional Sciences at the Intersection of Omics Disciplines and Ethics. Available online: http://onlinelibrary.wiley.com/doi/10.1002/9781118930458.ch47/summary (accessed on 11 March 2016).
- Page, A.J.; Reid, S.A.; Speedy, D.B.; Mulligan, G.; Thompson, J. Exercise-associated hyponatremia, renal function, and nonsteroidal antiinflammatory drug use in an ultraendurance mountain run. Clin. J. Sport Med. 2007, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bassini-Cameron, A.; Monteiro, A.; Gomes, A.; Werneck-de-Castro, J.P.S.; Cameron, L. Glutamine protects against increases in blood ammonia in football players in an exercise intensity-dependent way. Br. J. Sports Med. 2008, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Spreng, T.; Lehr, M.; Yang, B.; Karau, A.; Gebhardt, G.; Steinacker, G.M. The supportive effect of supplementation with α-keto acids on physical training in type 2 diabetes mellitus. Food Funct. 2015, 6, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Dalsgaard, M.K.; Steensberg, A.; Møller, K.; Secher, N.H. Cerebral ammonia uptake and accumulation during prolonged exercise in humans. J. Physiol. 2005, 563 Pt 1, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Secher, N.H. Cerebral perturbations provoked by prolonged exercise. Prog. Neurobiol. 2004, 72, 223–261. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Parnas, J.K. Ammonia formation in muscle and its source. Am. J. Physiol. 1929, 90, 467. [Google Scholar]
- Lowenstein, J.M. The purine nucleotide cycle revisited. Int. J. Sports Med. 1990, 11, S37–S46. [Google Scholar] [CrossRef] [PubMed]
- Rundell, K.W.; Tullson, P.C.; Terjung, R.L. AMP deaminase binding in contracting rat skeletal muscle. Am. J. Physiol. 1992, 263, C287–C293. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Morisaki, T.; Kamma, H.; Sugama, T.; Morisaki, H.; Ohtahara, A.; Holmes, E.W. Control of AMP deaminase 1 binding to myosin heavy chain. Am. J. Physiol. 1998, 275, C870–C881. [Google Scholar] [PubMed]
- Hellsten, Y.; Richter, E.A.; Kiens, B.; Bangsbo, J. AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J. Physiol. 1999, 520, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Babij, P.; Matthews, S.M.; Rennie, M.J. Changes in blood ammonia, lactate and amino acids in relation to workload during bicycle ergometer exercise in man. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Clancy, T.R.; Cook, J.R. Blood lactate and ammonium ion accumulation during graded exercise in humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 135–139. [Google Scholar] [PubMed]
- Kasperek, G.J.; Dohm, G.L.; Snider, R.D. Activation of branched-chain keto acid dehydrogenase by exercise. Am. J. Physiol. 1985, 248 Pt 2, R166–R171. [Google Scholar] [PubMed]
- Wagenmakers, A.J.; Coakley, J.H.; Edwards, R.H. Metabolism of branched-chain amino acids and ammonia during exercise: Clues from McArdle’s disease. Int. J. Sports Med. 1990, 11, S101–S113. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; van der Vusse, G.J.; Söderlund, K.; Wagenmakers, A.J. Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J. Physiol. 1995, 489, 251–261. [Google Scholar] [CrossRef]
- Felipo, V.; Butterworth, R.F. Neurobiology of ammonia. Prog. Neurobiol. 2002, 67, 259–279. [Google Scholar] [CrossRef]
- Camerino, S.R.A.S.; Lima, R.C.P.; França, T.C.L.; de Azevedo Herculano, E.; Rodrigues, D.S.A.; Rodrigues, D.A.S.; de Sousa Gouveia, M.G.; Cameron, L.C.; Prado, E.S. Keto analogues and amino acid supplementation and its effects on ammonemia and performance under thermoneutral conditions. Food Funct. 2016, 7, 872–870. [Google Scholar] [CrossRef] [PubMed]
- Pardue, H.L. A comprehensive classification of kinetic methods of analysis used in clinical chemistry. Clin. Chem. 1977, 23, 2189–2201. [Google Scholar] [PubMed]
- Werner, M.; von Wasielewski, R.; Komminoth, P. Antigen retrieval, signal amplification and intensification in immunohistochemistry. Histochem. Cell Biol. 1996, 105, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, G.; Steck, T.L.; Wallach, D.F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 1971, 10, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [PubMed]
- Bayer, E.; Grom, E.; Kaltenegger, B.; Uhmann, R. Separation of amino acids by high performance liquid chromatography. Anal. Chem. 1976, 48, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Kohen, F.; Pazzagli, M.; Kim, J.B.; Lindner, H.R. An immunoassay for plasma cortisol based on chemiluminescence. Steroids 1980, 36, 421–437. [Google Scholar] [CrossRef]
- Kumae, T.; Kurakake, S.; Arakawa, H.; Uchiyama, I. A study for prevention of chronic fatigue. part 2. effects of strenuous physical exercise performed in a training camp on serum enzyme activity levels and subjective fatigue. Environ. Health Prev. Med. 1998, 3, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Garbutt, G.; Lopes, P.; Pedoe, D.T. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br. J. Sports Med. 2004, 38, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.M.; Dantas, E.; de Salles, B.F.; Miranda, H.; Koch, A.; Willardson, J.M.; Simão, R. Creatine kinase and lactate dehydrogenase responses after upper-body resistance exercise with different rest intervals. J. Strength Cond. Res. 2010, 24, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J.; Verbalis, J.G.; Clement, S.; Mendelson, J.H.; Mello, N.K.; Adner, M.; Shirey, T.; Glowacki, J.; Lee-Lewandrowski, E.; Lewandrowski, K.B. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am. J. Med. 2007, 120, 461.e11–461.e17. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Hsieh, S.S. Effects of a 7-day eccentric training period on muscle damage and inflammation. Med. Sci. Sports Exerc. 2001, 33, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Milias, G.A.; Nomikos, T.; Fragopoulou, E.; Athanasopoulos, S.; Antonopoulou, S. Effects of eccentric exercise-induced muscle injury on blood levels of platelet activating factor (PAF) and other inflammatory markers. Eur. J. Appl. Physiol. 2005, 95, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, A.; Fatouros, I.; Gourgoulis, V.; Avloniti, A.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Michailidis, Y.; Beneka, A.; Malliou, P.; et al. Time course of changes in performance and inflammatory responses after acute plyometric exercise. J. Strength Cond. Res. 2010, 24, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Watanabe, H.; Kishihara, C.; Taniguchi, N.; Takakuwa, E. Effect of physical exercise on the activity of GOT isozyme in human plasma. Tohoku J. Exp. Med. 1978, 126, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K.E.; Sivyer, G.; Sivyer, K.; Dare, A. The biochemistry of runners in a 1600 km ultramarathon. Br. J. Sports Med. 1999, 33, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 09502. [Google Scholar] [CrossRef] [PubMed]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [PubMed]
- Syu, G.D.; Chen, H.I.; Jen, C.J. Differential effects of acute and chronic exercise on human neutrophil functions. Med. Sci. Sports Exerc. 2012, 44, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Syu, G.D.; Chen, H.I.; Jen, C.J. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PLoS ONE 2011, 6, e24385. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.; Gleeson, M.; Woods, J.A.; Bishop, N.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goete, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Castell, L.M.; Newsholme, E.A. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997, 13, 738–742. [Google Scholar] [CrossRef]
- Castell, L.M.; Newsholme, E.A. The relation between glutamine and the immunodepression observed in exercise. Amino Acids 2001, 20, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Poortmans, J.R.; Leclercq, R.; Brasseur, M.; Duchateau, G.; Newsholme, E.A. Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Block, K.P.; Repa, J.J.; Torres, N.; Nawabi, M.D.; Buse, M.G.; Harper, A.E. High branched-chain alpha-keto acid intake, branched-chain alpha-keto acid dehydrogenase activity, and plasma and brain amino acid and plasma keto acid concentrations in rats. Am. J. Clin. Nutr. 1990, 52, 313–319. [Google Scholar] [PubMed]
- Rohde, T.; MacLean, D.A.; Pedersen, B.K. Effect of glutamine supplementation on changes in the immune system induced by repeated exercise. Med. Sci. Sports Exerc. 1998, 30, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Gelly, K.; Jackman, M.L.; Patel, A.; Simeoni, M.; Rennie, M.J. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J. Appl. Physiol. 1999, 86, 1770–1777. [Google Scholar] [PubMed]
- Banister, E.W.; Cameron, B.J. Exercise-induced hyperammonemia: Peripheral and central effects. Int. J. Sports Med. 1990, 11, S129–S142. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K.; Harris, R.C.; Nylind, B.; Hultman, E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976, 367, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Few, J.D. Effect of exercise on the secretion and metabolism of cortisol in man. J. Endocrinol. 1974, 62, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.A.; Garthwaite, T.L.; Gustafson, A.B. Plasma adrenocorticotropin and cortisol responses to submaximal and exhaustive exercise. J. Appl. Physiol. 1983, 55, 1441–1444. [Google Scholar] [PubMed]
- Duclos, M.; Gouarne, C.; Bonnemaison, D. Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J. Appl. Physiol. 2003, 94, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M. Hepatic glucose production during exercise. Adv. Exp. Med. Biol. 1998, 441, 117–127. [Google Scholar] [PubMed]
- Kruger, K.; Agnischock, S.; Lechtermann, A.; Tiwari, S.; Mishra, M.; Pilat, C.; Wagner, A.; Tweddell, C.; Gramlich, I.; Mooren, F.C. Intensive resistance exercise induces lymphocyte apoptosis via cortisol and glucocorticoid receptor-dependent pathways. J. Appl. Physiol. 2011, 110, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Price, T.B.; Bergeron, R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: Impact of type 1 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4656–4664. [Google Scholar] [CrossRef] [PubMed]
- Viru, A.; Karelson, K.; Smirnova, T. Stability and variability in hormonal responses to prolonged exercise. Int. J. Sports Med. 1992, 13, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M.N.; Franchini, M.; Molinaro, S. Bridging the gap between translational medicine and unmet clinical needs. Technol. Health Care 2015, 23, 109–118. [Google Scholar] [PubMed]
- Magno-Franca, A.; Bassini, A.; Bessa, A.O.; Nissenbaum, M.; Vaz de Macedo, D.; Cameron, L.C. A sportomic follow-up of a muscle injury succeeded by acetaminophen hepatotoxicity. OA Sports Med. 2013, 1, 1–5. [Google Scholar] [CrossRef]





| Anthropometry | |
|---|---|
| Height (m) | 1.77 ± 0.02 |
| Weight (Kg) | 82.9 ± 5.0 |
| Fat weight (Kg) | 9.8 ± 2.4 |
| Fat-free mass (Kg) | 73.0 ± 2.6 |
| Fat percentage (%) | 11.5 ± 2.0 |
| BMI (Kg/m2) | 26.2 ± 1.2 |
| Lipid Panel (mg/dL) | |
|---|---|
| Serum cholesterol | 173.6 ± 24.2 |
| Serum triacylglycerol | 83.3 ± 20.0 |
| HDL | 55.3 ± 5.0 |
| LDL | 95.0 ± 26.5 |
| VLDL | 16.6 ± 3.8 |
| Cholesterol/HDL ratio | 3.2 ± 0.7 |
| LDL/HDL ratio | 1.9 ± 0.6 |
| Non cholesterol lipids | 118.3 ± 28.9 |
| Protein Fractions (g/dL) | |
|---|---|
| Total proteins | 6.7 ± 0.12 |
| Albumin | 2.7 ± 1.23 |
| α-1 globulin | 0.4 ± 0.14 |
| α-2 globulin | 0.5 ± 0.05 |
| β-1 globulin | 0.4 ± 0.02 |
| β-2 globulin | 0.2 ± 0.003 |
| γ globulin | 1.2 ± 0.12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, W.S.; Viveiros de Castro, L.; Deane, E.; Magno-França, A.; Bassini, A.; Cameron, L.-C. Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach. Nutrients 2016, 8, 719. https://doi.org/10.3390/nu8110719
Coelho WS, Viveiros de Castro L, Deane E, Magno-França A, Bassini A, Cameron L-C. Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach. Nutrients. 2016; 8(11):719. https://doi.org/10.3390/nu8110719
Chicago/Turabian StyleCoelho, Wagner Santos, Luis Viveiros de Castro, Elizabeth Deane, Alexandre Magno-França, Adriana Bassini, and Luiz-Claudio Cameron. 2016. "Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach" Nutrients 8, no. 11: 719. https://doi.org/10.3390/nu8110719
APA StyleCoelho, W. S., Viveiros de Castro, L., Deane, E., Magno-França, A., Bassini, A., & Cameron, L.-C. (2016). Investigating the Cellular and Metabolic Responses of World-Class Canoeists Training: A Sportomics Approach. Nutrients, 8(11), 719. https://doi.org/10.3390/nu8110719





